Properties of Solids Review
5.0(1)
5.0(1)
Card Sorting
1/35
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
1
New cards
What is the bond angle of sp?
180
2
New cards
What is the bond angle of sp2?
120
3
New cards
What is the bond angle of sp3?
109\.5
4
New cards
What is the bond angle of sp3d?
120 between atoms on the x axis, 90 between x axis and y axis molecules
5
New cards
What is the bond angle between sp3d2?
90 between all
6
New cards
Which atoms are electron deficient?
Everything left of Carbon (besides Lithium)
7
New cards
What does VSEPR stand for?
Valence Shell Electron Pair Repulsion
8
New cards
What is VSEPR theory?
All pairs of e- on the valence shell repel as far apart as possible.
9
New cards
What is the EN range of non polar covalent bonds?
0\.0-0.5
10
New cards
What is the EN range of polar covalent bonds?
0\.5-1.7
11
New cards
What is the EN range of ionic bonds?
1\.7-4.0
12
New cards
A molecule will be non-polar if…
It has all non-polar bonds or is symmetrical so dipoles cancel.
13
New cards
A molecule will be polar if…
It has polar bonds AND is non-symmetrical so dipoles do not cancel.
14
New cards
What does intra mean (as in intramolecular)?
Intra=within
15
New cards
What does inter mean (as in intermolecular)?
Inter=between
16
New cards
List the vanderwaal forces
Dip-dip, London dispersion, hydrogen bonding
17
New cards
What are dip-dip bonds?
Bond that occurs between a positive end of one polar molecule and a negative end of another. 1% as strong as covalent.
18
New cards
What are H-bonds?
Occurs between a Hydrogen bonded to a high EN atom and a partially negative atom on another molecule. 10-20x weaker than covalent.
19
New cards
What are London dispersion forces?
A force that exists between non-polar molecules, this increases as molecular mass increases. The weakest force.
20
New cards
Which forces create high m.p, high b.p, high surface tension and high viscosity?
Dip-dip and h-bonding
21
New cards
What is surface tension?
The resistance of a liquid to increase its surface area. Liquids with large intermolecular forces have high surface tension.
22
New cards
What is capillary action?
The ability of a liquid to rise in a narrow tube. Caused by cohesive and adhesive forces.
23
New cards
What is the difference between cohesive and adhesive forces?
Cohesive- intermolecular forces within entities of liquids.
Adhesive- forces between liquid and the container.
Adhesive- forces between liquid and the container.
24
New cards
Is Ionic bonding stronger or weaker than all intermolecular forces?
Stronger
25
New cards
Properties of Ionic Solids:
1. Hard but brittle
2. Conduct electricity in liquid state or when in solutions
3. High m.p
26
New cards
Define Ionic solids:
Crystal structure of ions held together by strong directional ionic bonds.
27
New cards
What are metallic crystals?
Metals have low ionization energy so they do not form ionic bonds with themselves or with other metals. They hold electrons loosely and share them.
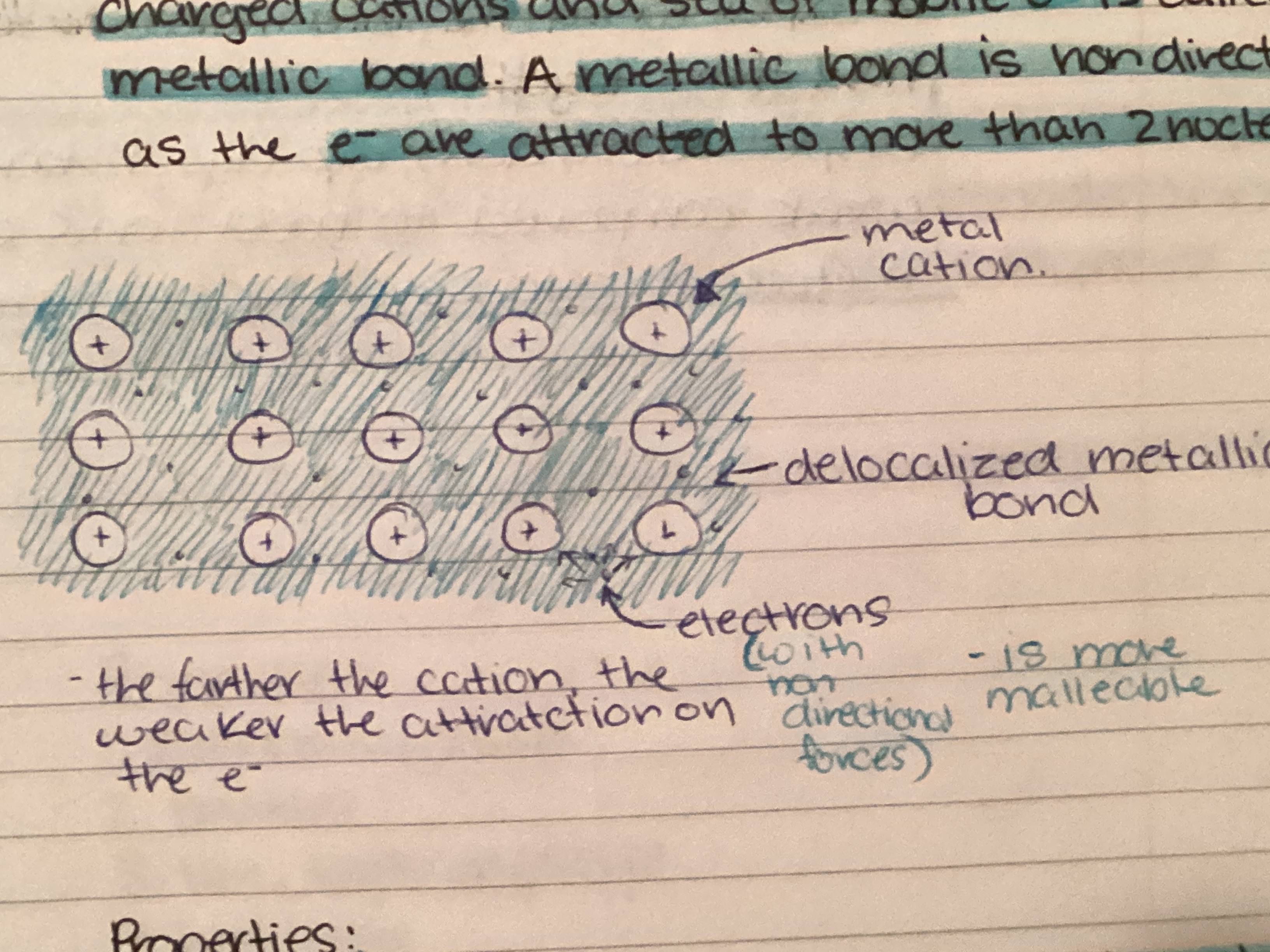
28
New cards
Properties of metallic crystals:
1. Conduct electricity
2. Malleable and ductile
3. High m.p and b.p
29
New cards
Define small molecular crystals:
Crystals of elements or compounds
30
New cards
Properties of small molecular crystals:
1. Low m.p and b.p
2. Not very hard
3. Non-conductors
31
New cards
Why do properties vary between metallic and small molecular crystals?
Because metallic crystals cannot form ionic bonds this causes it to use dip-dip and h-bonding which creates increased m.p and b.p.
32
New cards
Define Covalent network crystals:
Atoms joined by strong directional covalent bonds in a covalent network.
33
New cards
Explain diamond:
Each Carbon is sp3 hybridized to adjacent carbon. It is a large molecule, covalent bonds hold it together no electrons are localized.
34
New cards
Explain graphite:
Each Carbon is bonded to 3 sp3 hybridized carbon. It has a delocalized structure that conducts.
35
New cards
Properties of covalent network crystals:
1. Extremely Hard
2. Brittle
3. Very high m.p and b.p
4. Insoluble
5. Non conductors (except graphite)
36
New cards