L2 18 electron rule and MLXZ Formalism
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
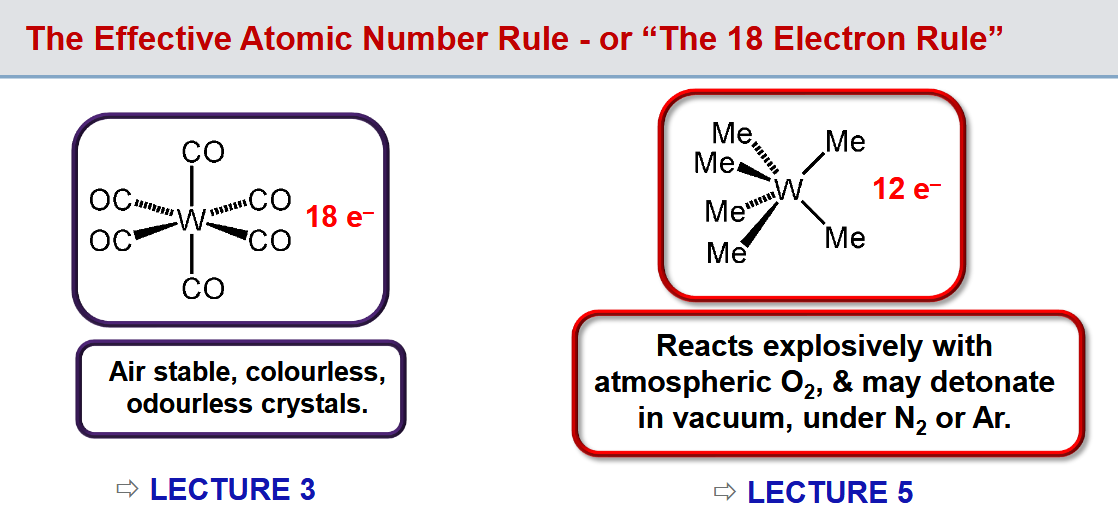
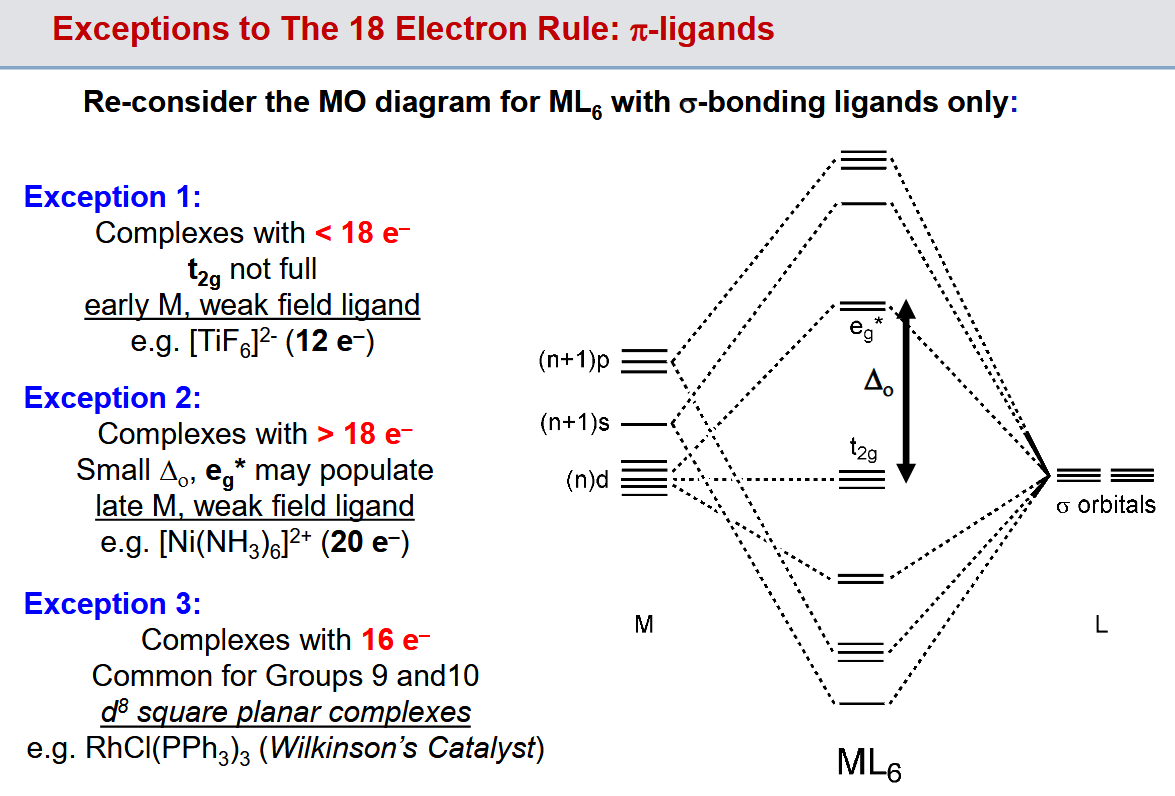
What is the 18 electron rule
Stable organometallic compounds of transition metals tend to have a total of 18 electrons around the metal.
Other electron counts can exist though
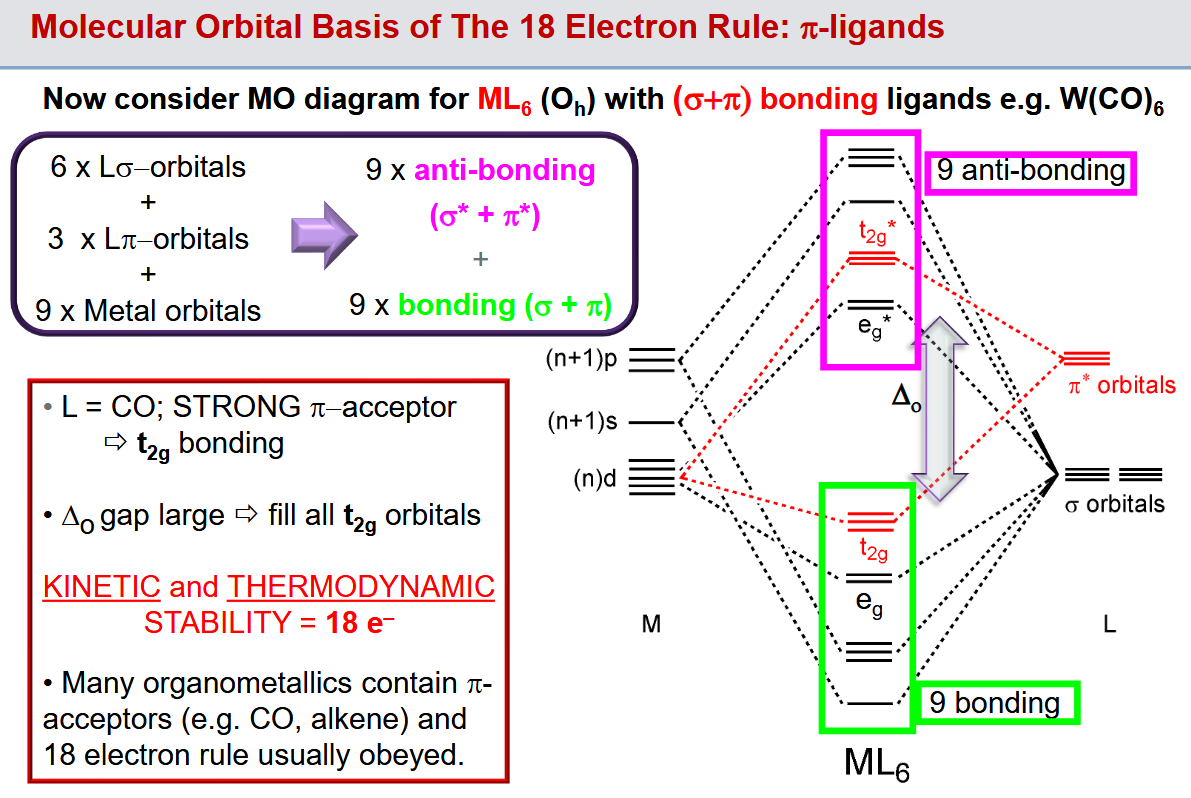
Why is 18 electrons preferred
The idea of just filling the 18 electron shell is a massive oversimplification.
Electron counts of 18 (sometimes 12) are observed due to the filling of the 6 bonding eg orbitals and the 3 t2g orbitals which are made bonding thanks to stabilisation from the inclusion of pi donor ligands
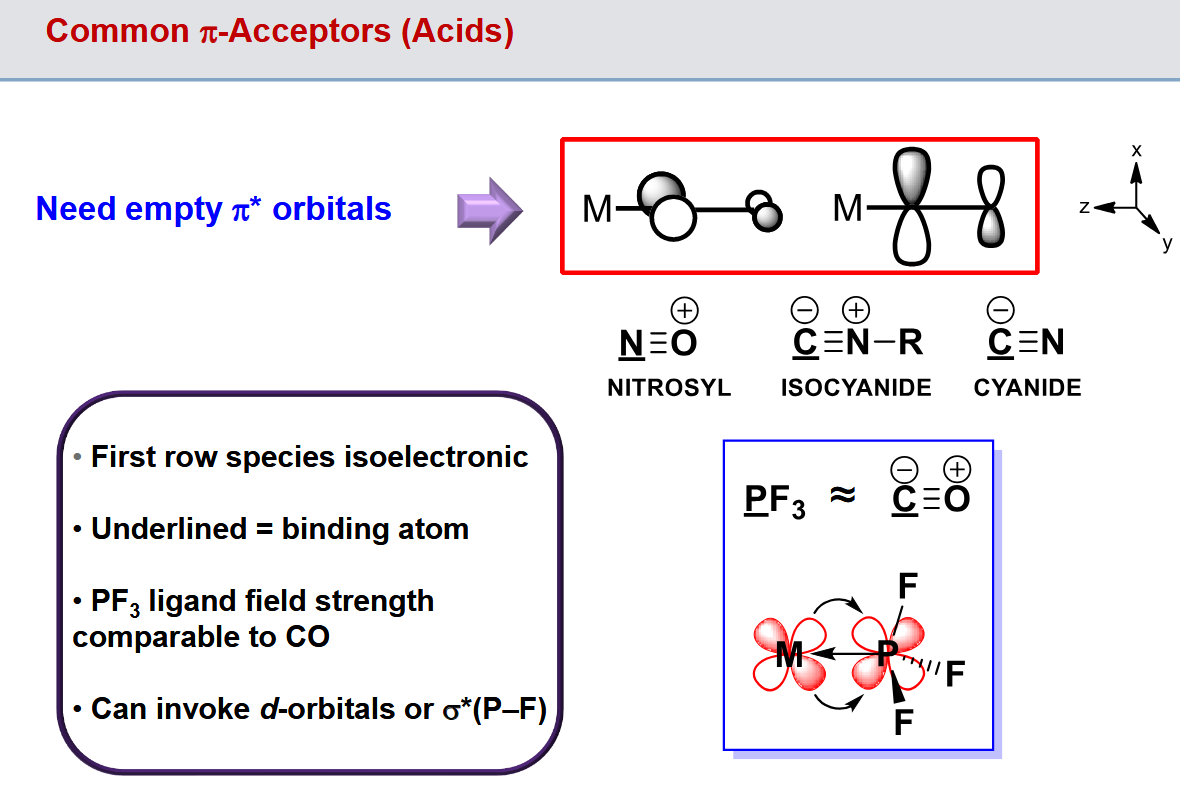
Features of pi acceptor ligands
18 electron rule exceptions
Main one is square planar complexes in which we only have 2 non-bonding orbitals thanks to the eg and t2g orbitals “swapping” energies
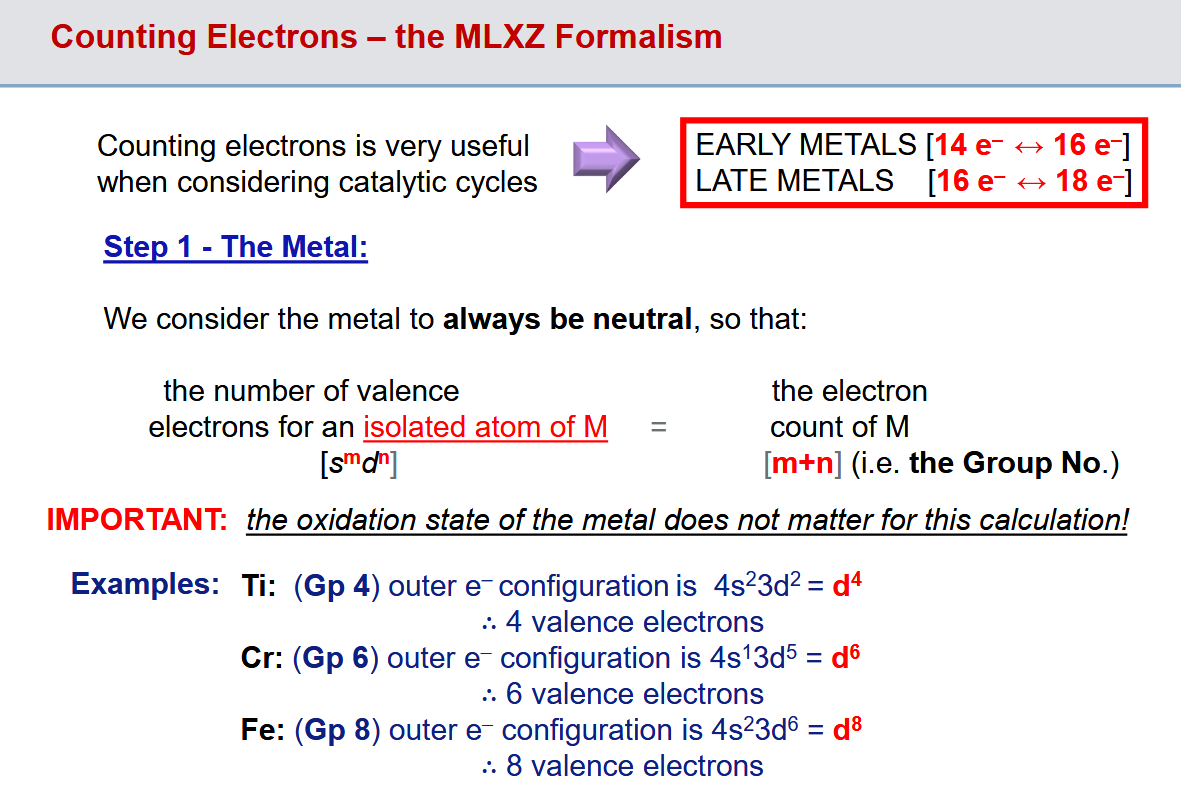
MLXZ Formalism - Step 1 for electron counting: the metal
Literally just the group number = d count
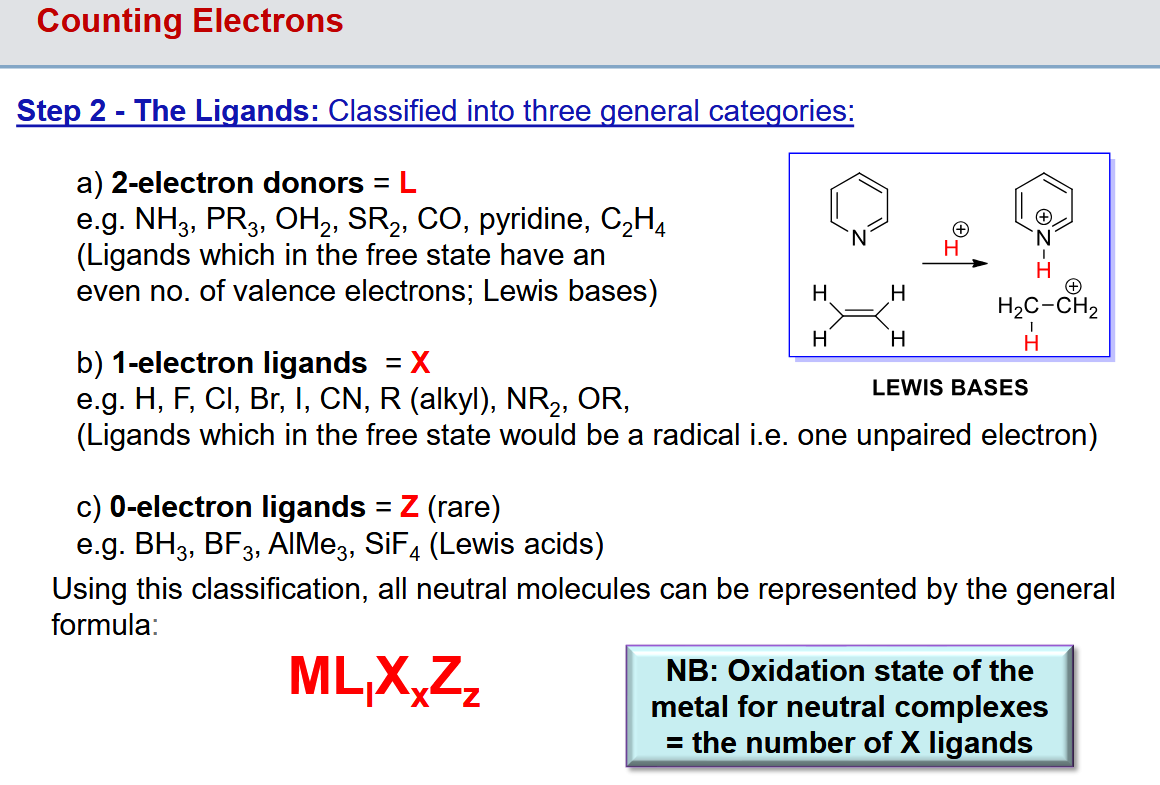
Step 2: Classifying the ligands
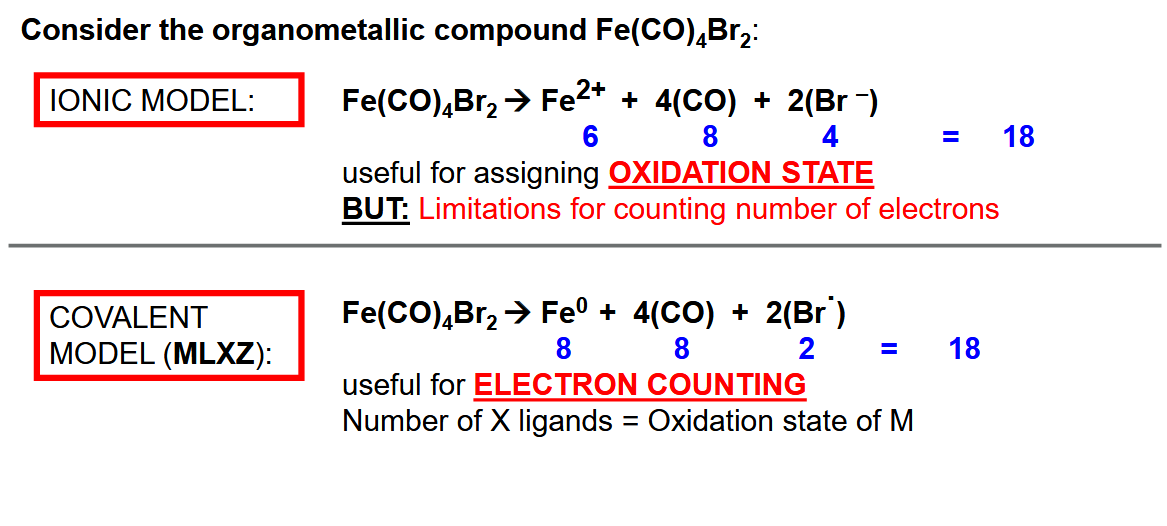
Ionic vs covalent model for counting electrons (don’t need to know ionic model - recommended to use covalent)
What L/X notation does a cyclopentadienyl ligand have
L2X
Two alkene electron donors and a negative carbon ligand

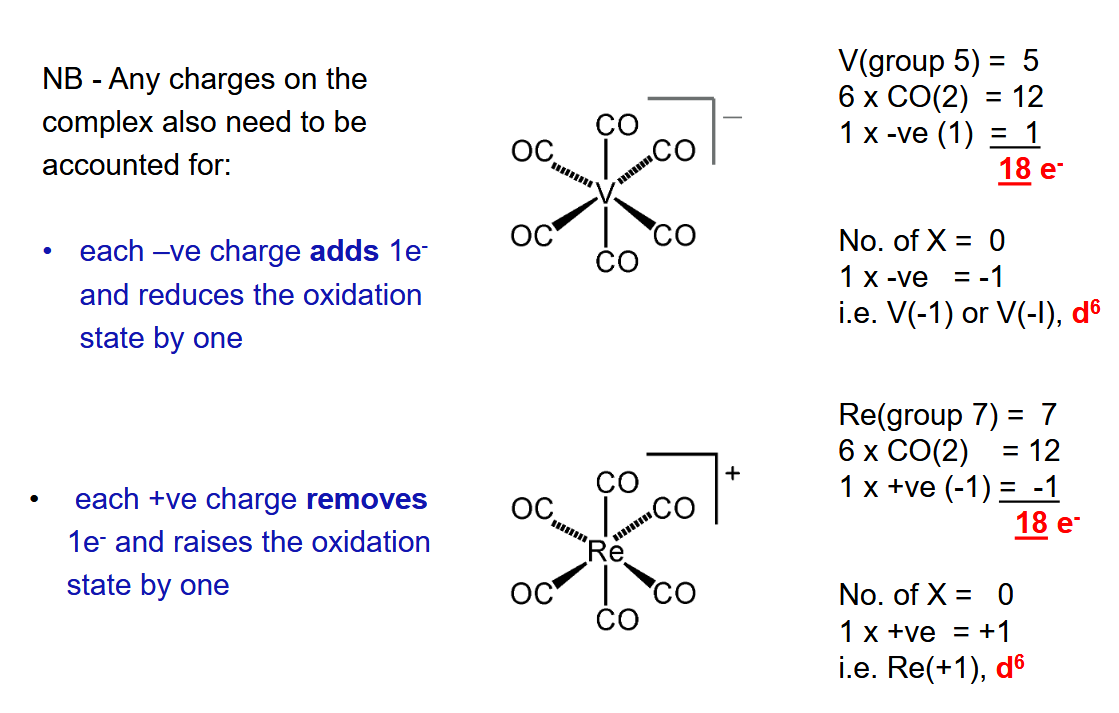
How do charged complexes work with electron counting and how does the oxidation state of the metal get affected
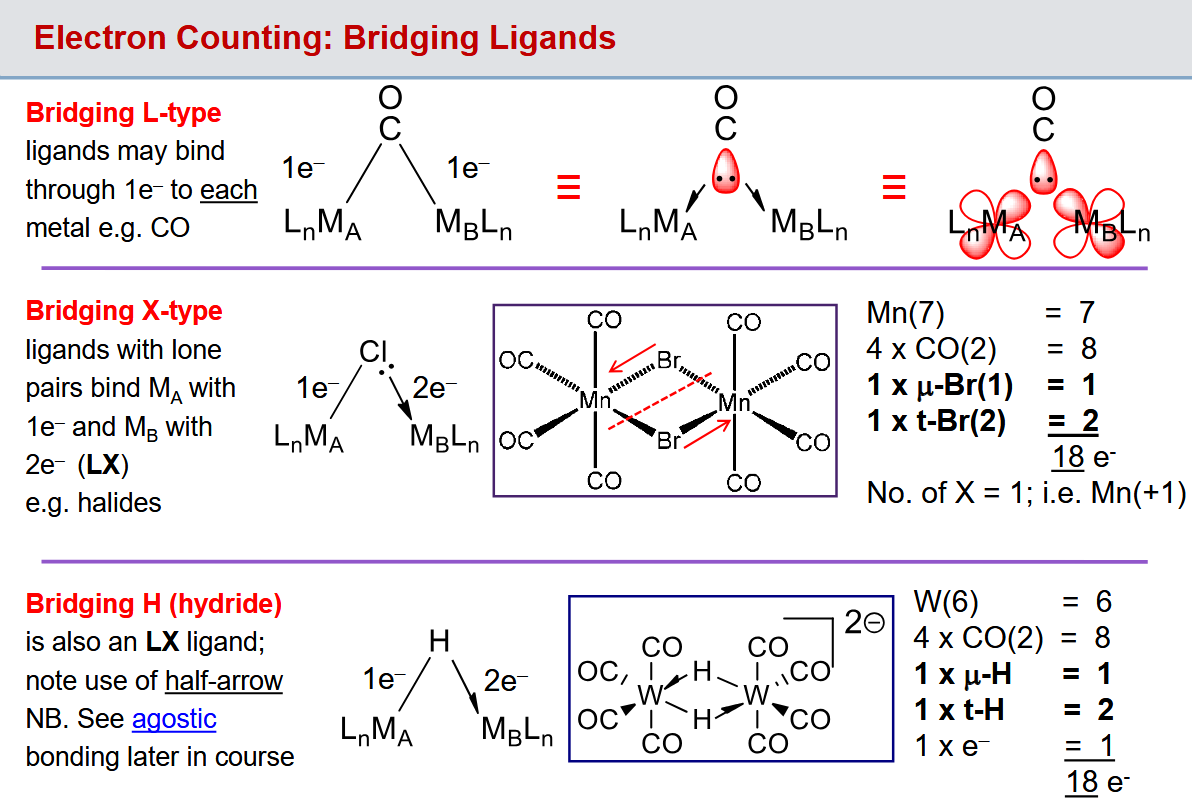
How are electrons counted in bridging L types, X types and hydrides
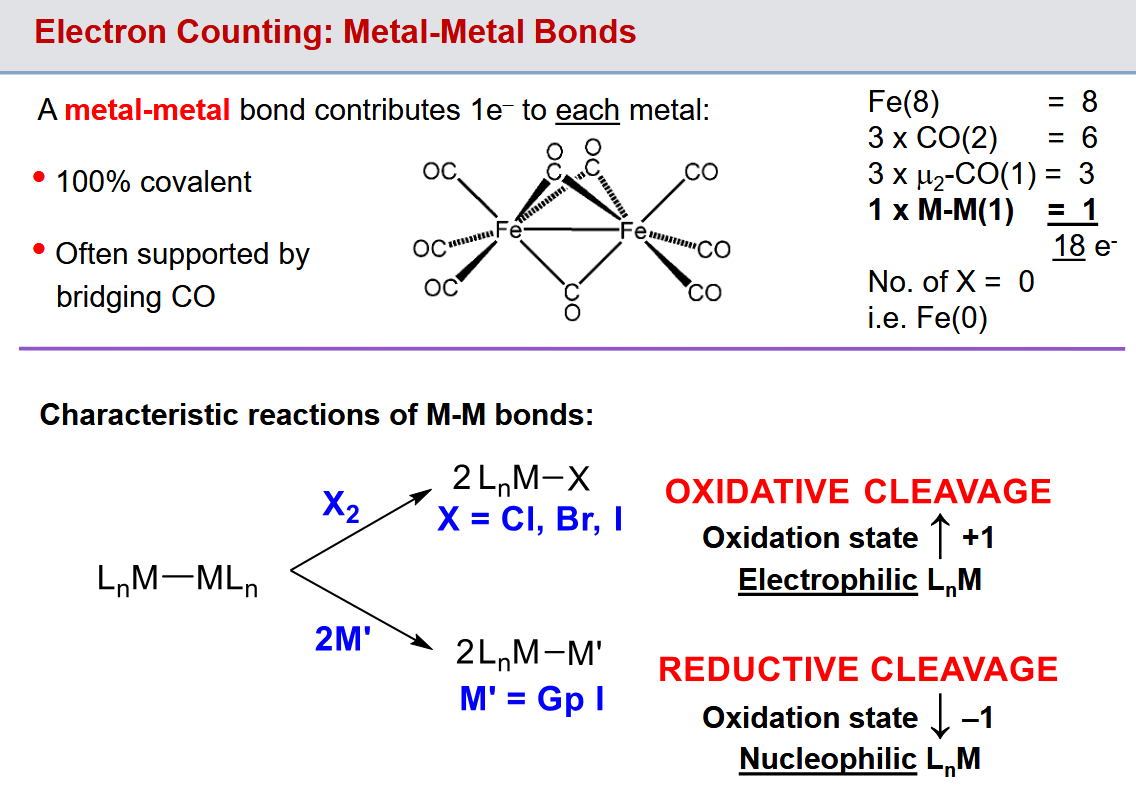
How are elctrons counted in Metal-Metal bonds
1e- to each metal
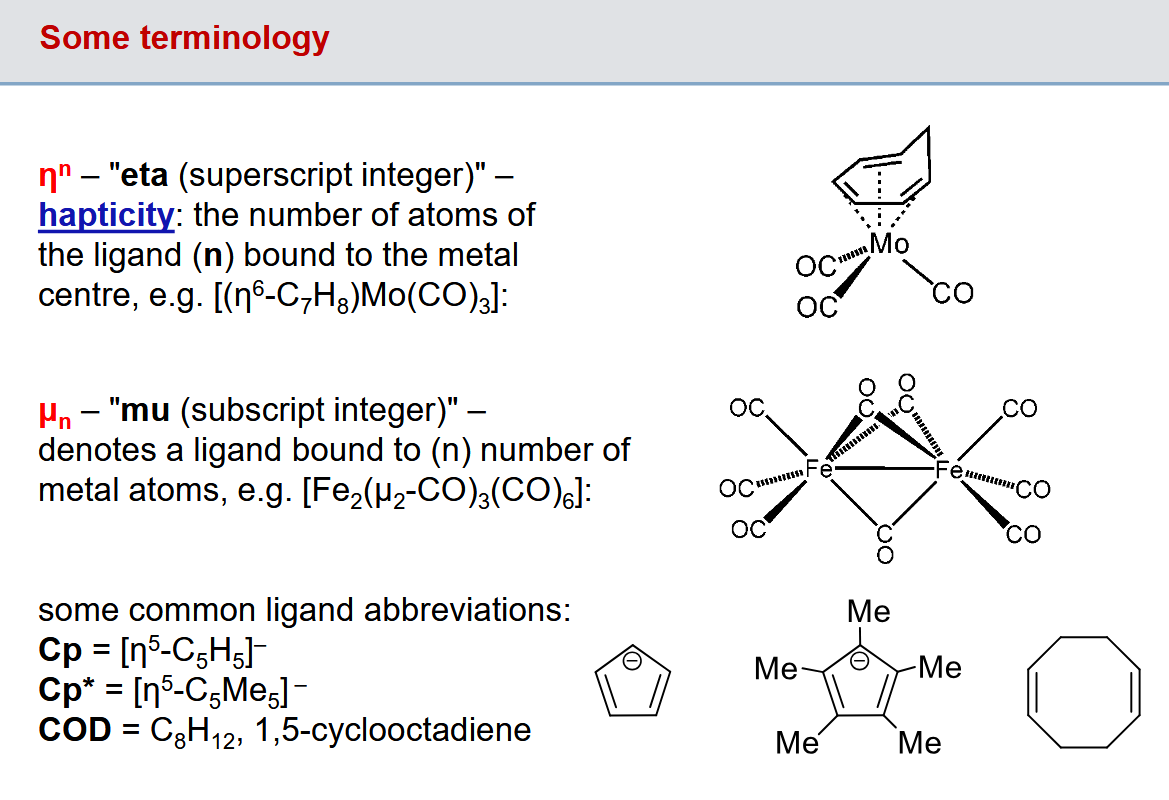
eta and mu terminlogy for hapticity and bridges
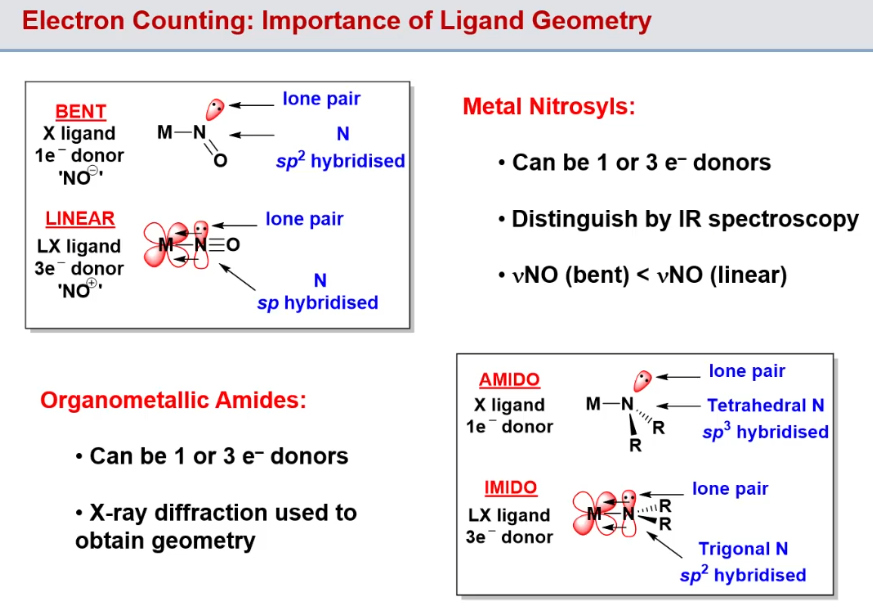
Metal Nitrosyls and organometallic amides - how does their geometry affect what kind of ligand they are
Note how nitrosyls are NO- or NO+ depending on its geometry