IMED1003 - Bioenergetics (L2)
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
1st Law of Thermodynamics
- Two fundemental laws govern thermodynamics
1st Law of Thermodynamics:
- Energy cannot be created or destroyed. Energy can be transferred and transformed

2nd Law of Thermodynamics
- Disorder (entropy) in universe is increasing
- Energy transformations proceed spontaneously (convert matter from more ordered and less stable to less ordered and more stable)
- Spontaneous changes that do not require outside energy increase entropy (disorder)
- For a process to occur without energy input, it must increase the entropy of the system
- basically if you just continue normally your room becomes messy. it takes energy to bring order to the room

Second Law of Thermodynamics More details
- during energy transfer or transformation, some energy is unusable, often lost as heat
- Heat = measure of random motion of molecules.
- cells convert organised forms of energy to heat
- According to the second law of thermodynamics: every energy transfer or transformation increases entropy (disorder) of the universe
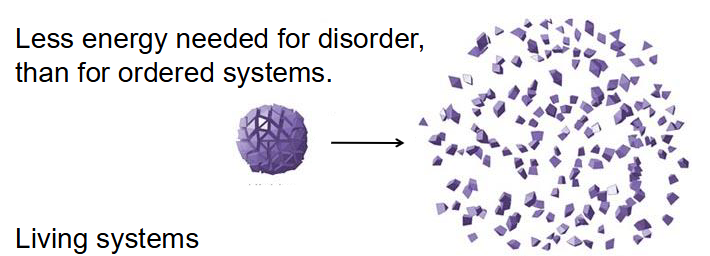
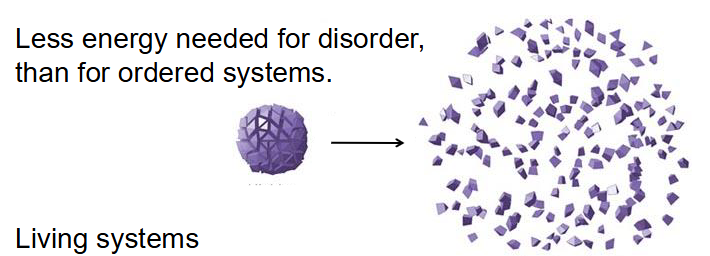
Life Requires a Lack of Entropy
- Less energy needed for disorder, then for ordered systems
- Living systems: Increase entropy of the universe, use energy to maintain order, have free energy to do work in cellular conditions
- Organisms live at the expense of "free energy"
- think about how proteins, made of many amino acids (disordered pieces) make one single protein (one ordered molecule)
Energy - Summary
- Energy: the ability to do work or cause change
- Kinetic: Motion
- Potential: stored
- Chemical: atoms/molecules
Summary of Laws of Thermodynamics:
1. Energy can be transferred and transformed, but it can't be created or destroyed
2. Every energy transfer or transformation increases the entropy of the universe
Anabolic Pathway of Metabolic Pathway
- anabolism: build complex molecules from simpler ones - consumes energy
Catabolic Pathway of Metabolic Pathway
- catabolism: break complex molecules into simpler ones, releases energy
Potential Energy
- stored energy (includes chemical energy in molecules)
Kinetic Energy
- currently causing change (involves some type of motion)
Gibbs' Free Energy
- in cells, molecules have certain amount of free energy (G)
- Gibbs' free energy (G) - energy contained in molecule's chemical bonds (when Temp and Press Constant)
- the free energy associated with a reaction = energy available for doing work
- Chemical reactions - change in free energy (change in G) (Delta G)
- Delta G can be positive or negative
- In test tube, some reactions release heat (exothermic), others absorb heat (endothermic)
- not all this energy available for chemical reactions - some transferred as heat, as entropy increases

Free Energy
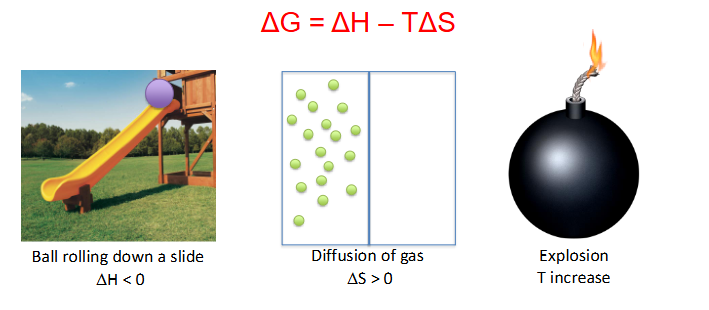
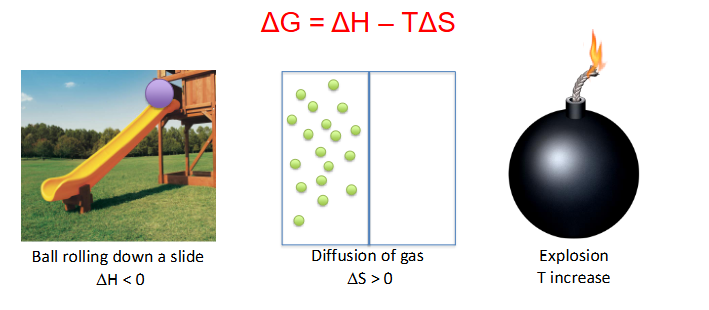
Delta G = Delta H minus (T * delta S)
- equation describes change in free energy when accounting for transfer of heat (enthalpy, H) and change in disorder (entropy, S) of the system (T = temperature)
- Enthalpy: measure of energy in thermodynamic system. We'll simply call it heat content, even though this isn't strictly accurate
- Entropy: amount of disorder. In the context of energy exchange, entropy = energy unavailable for use

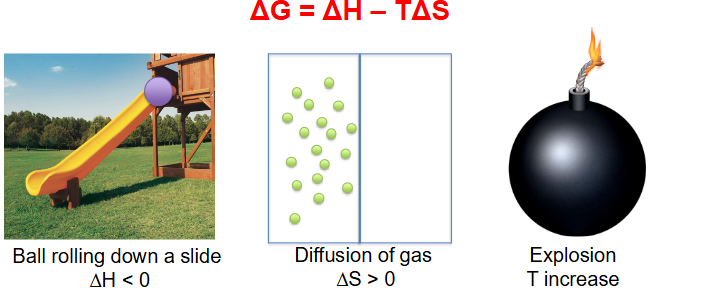
More on Free Energy
- the potential energy at the top is higher for the ball, so as it rolls down it loses potential energy hence delta H is negative
- Diffusion of gas increases disorganisation of particles hence entropy is greater then 0
- Explosion massively increases temperature
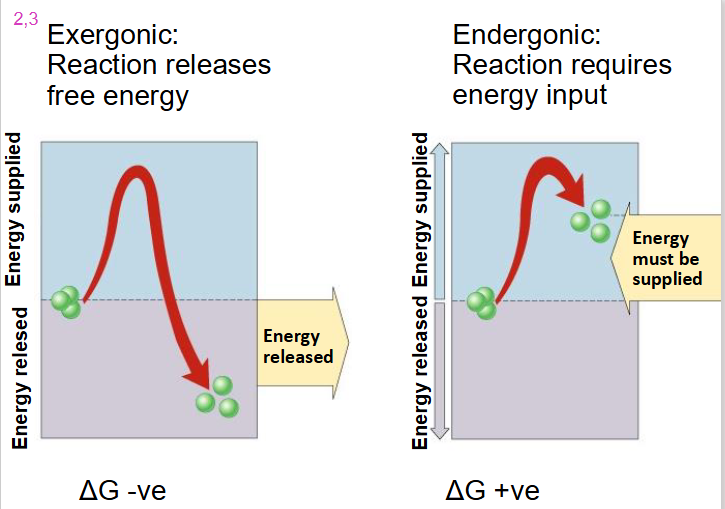
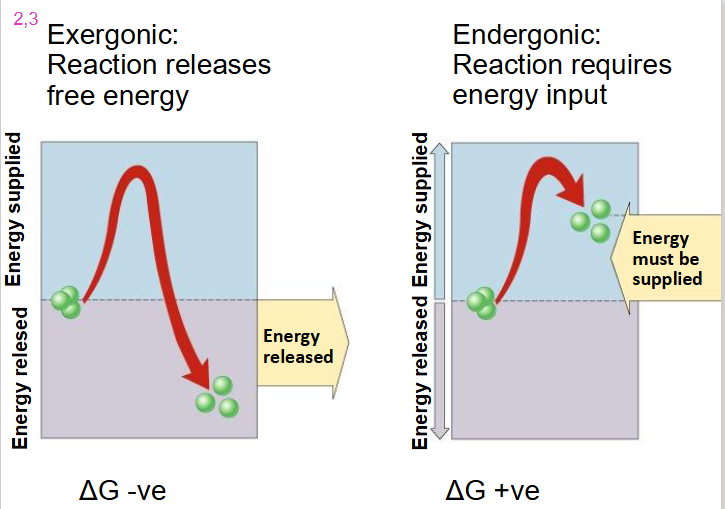
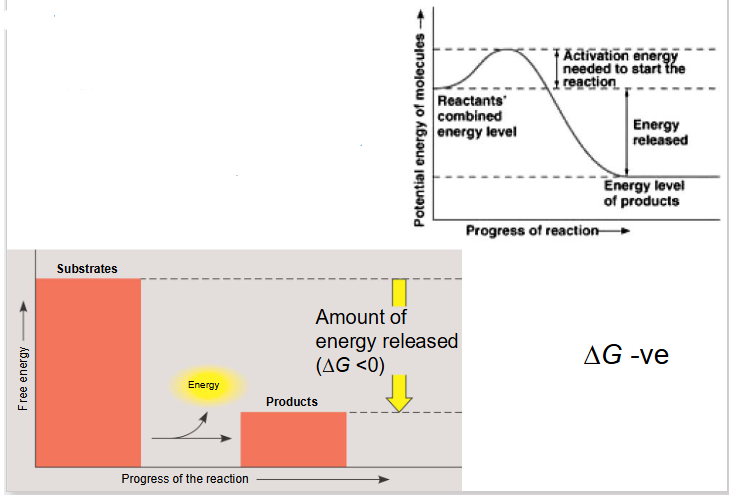
Exergonic Reaction
- reaction releases free energy
- delta G is negative
- we go from substrate with high level of energy in bonds to products with low level of energy in bonds with dissipation of heat
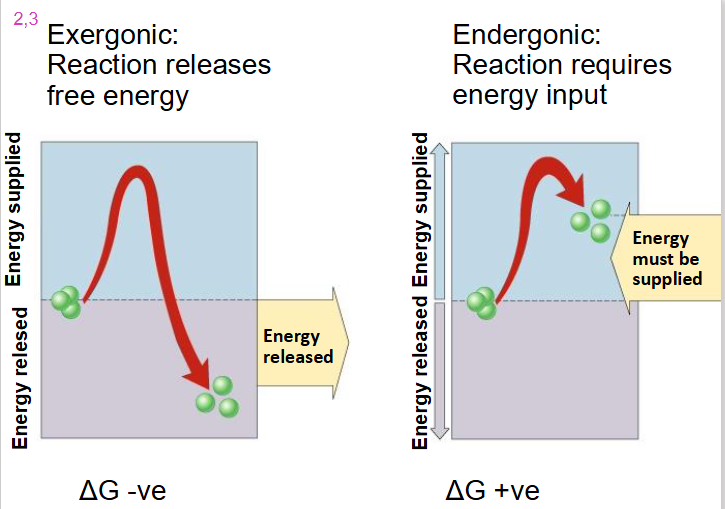
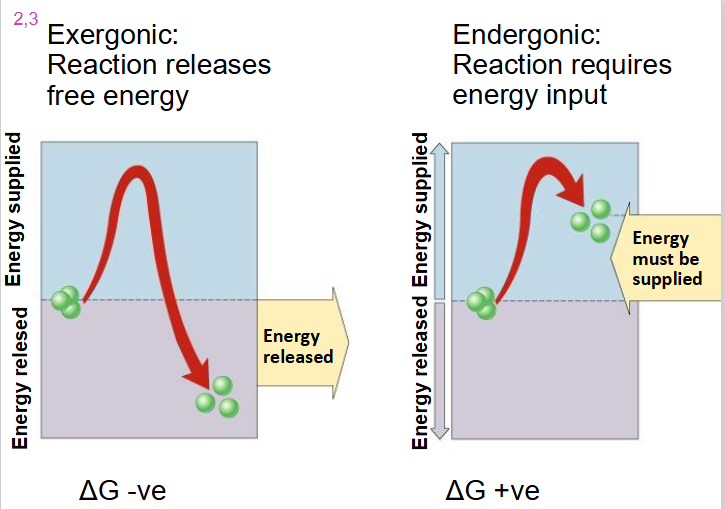
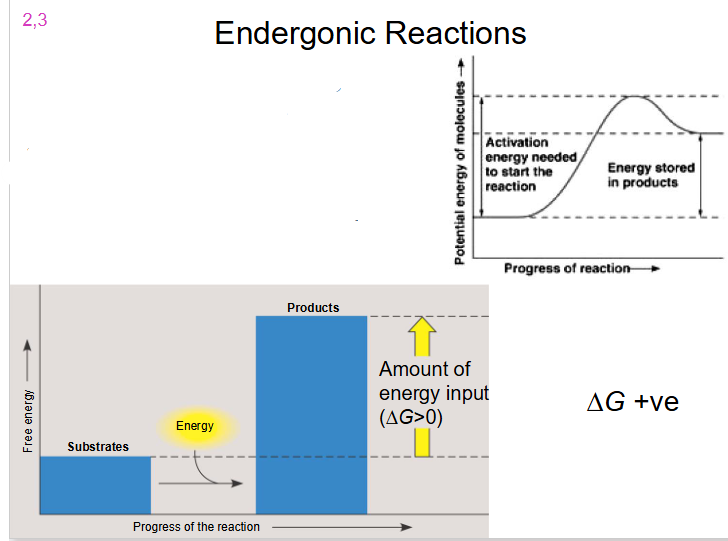
Endergonic Reaction
- reaction requires energy input
- delta G is positive
- substrate goes from low energy to high energy (energy added)
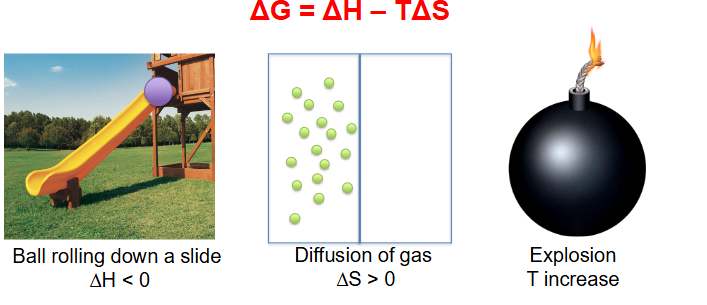
Delta G examples
- #1 has a Delta G of less then 0 (when ball rolls down slide T increase (friction))
- #2 has Delta G of less then 0 (since entropy increases)
- #3 has T increase so Delta G is less then 0
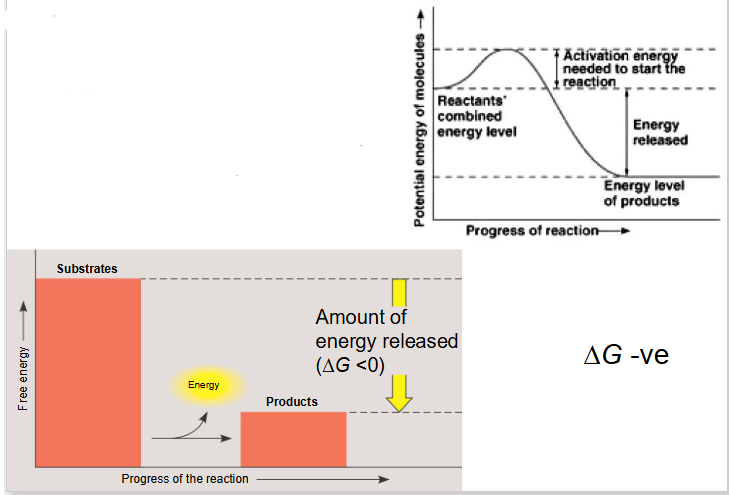
Exergonic Reactions More Info
- substrates have more free energy then products
- net release of energy and or increase of entropy
- occur spontaneously (without net input of energy)
- Delta G is negative
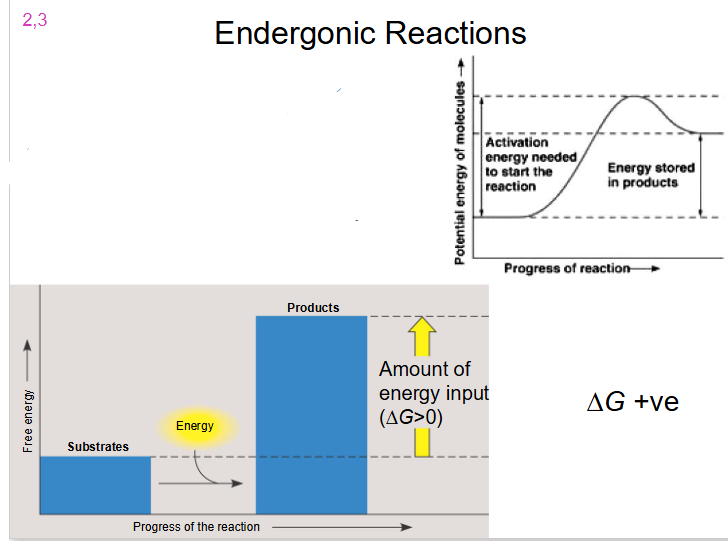
Endergonic Reactions
- Substrates have less free energy then products
- Net input of energy and or decrease in entropy
- do not occur spontaneously
- Delta G is positive
Energetics of Reactions
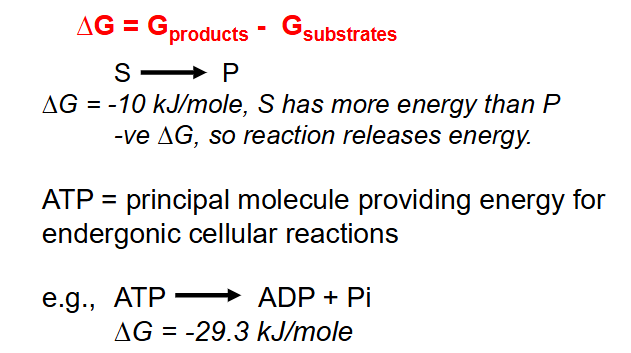
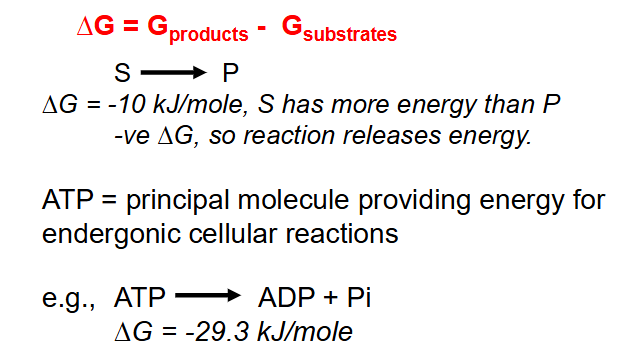
- when molecules (substrates) are altered to form new molecules (products), the energy change is given by:
- Delta G = G product - G substrates
DIAGRAM ON SLIDE 16
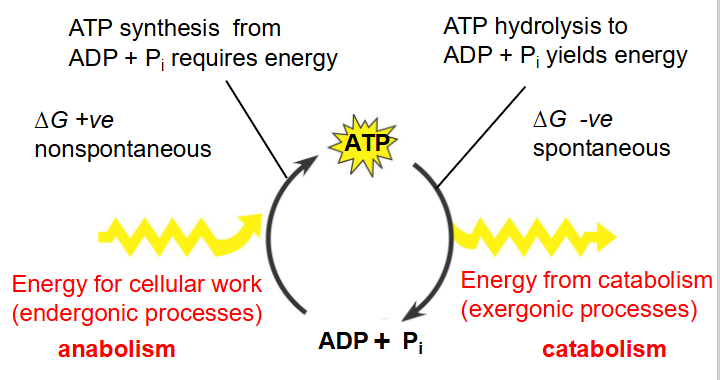
- ATP = principal molecule providing energy for endergonic cellular reactions
- e.g ATP --> ADP + Pi
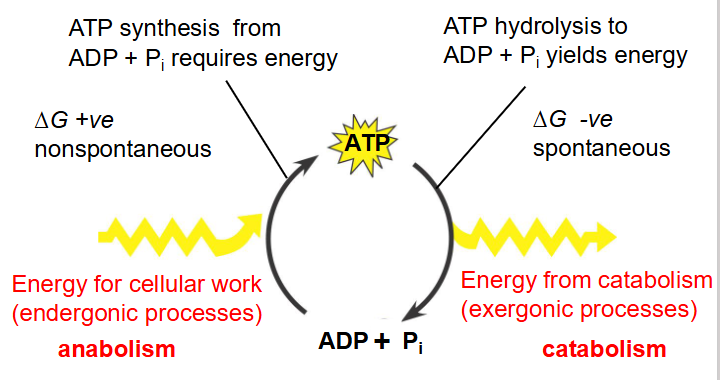
Energy Transfer in Cells
- endergonic: use energy
- exergonic: release energy
- Exergonic Reactions can be coupled to endergonic reactions
- ATP Synthesis from ADP + Pi requires energy
- Pi is inorganic phosphate
- basically ATP synthesis from ADP and Pi has delta G positive
- ATP hydrolysis has delta G negative
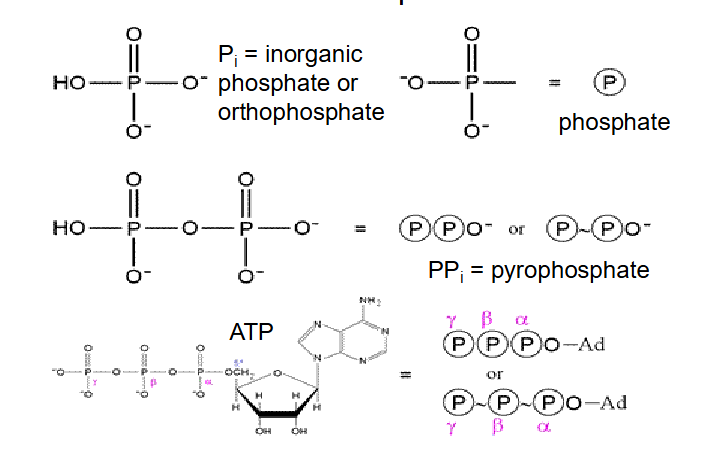
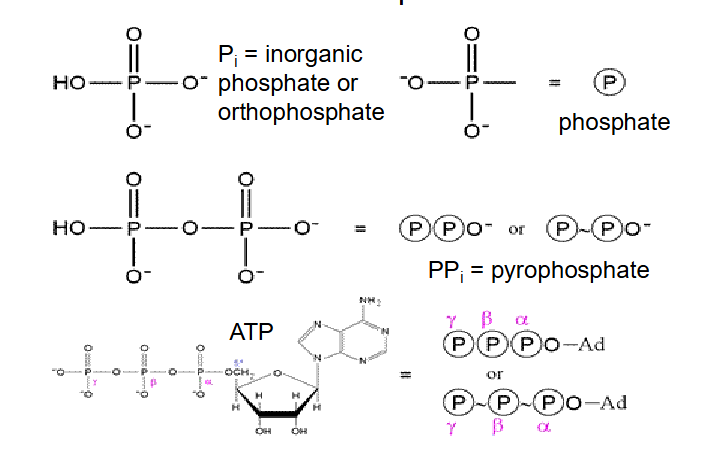
ATP and Phosphates
- Pi is inorganic phosphate
- PPi is pyrophosphate
we have phosphoanhydride bonds
- each ATP molecule can release energy when Pi is removed
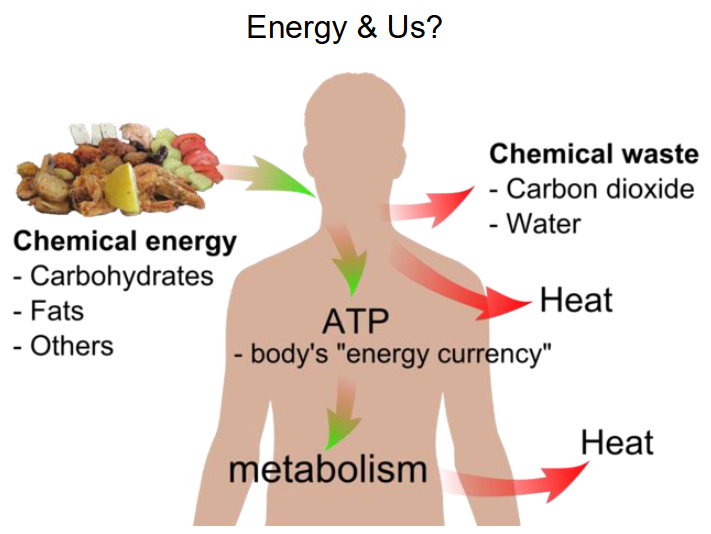
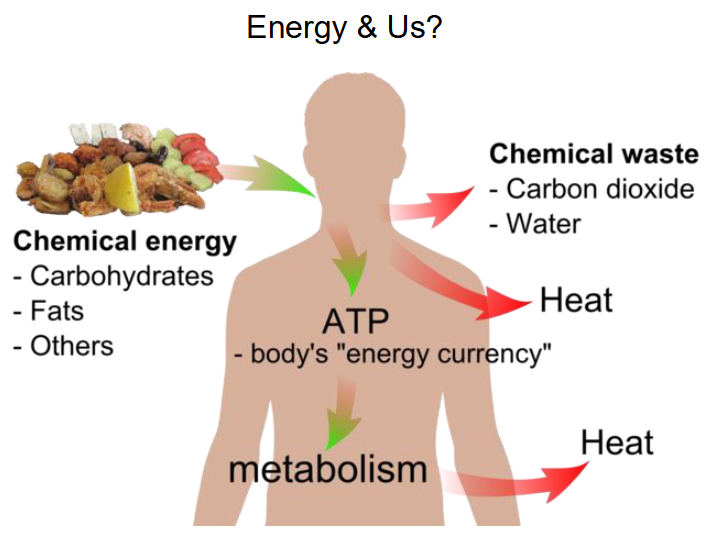
Energy and Us (NO LEARNING OUTCOME)
- chemical energy goes in (carbohydrates, fats, others)
- Chemical waste goes out (carbon dioxide, water)
- Heat goes out
- ATP is the body's energy currency
- Metabolism
ATP levels and usage
- estimated to be around 100g of ATP in healthy adults, some estimates up to 250g, not that much
- We use around 70kg ATP/day. So we have to generate ATP, recycle and reuse the core components
- Cells need 1-10mM ATP to function
- cells have a pool of ATP interconverted to ADP and AMP (and again, again, again and so on)
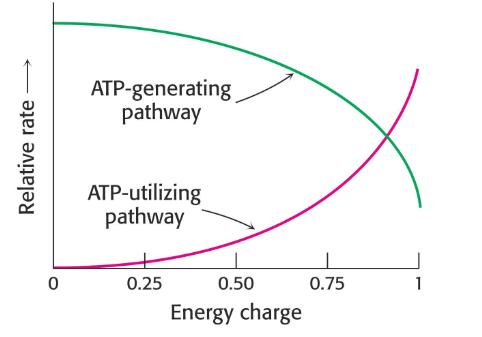
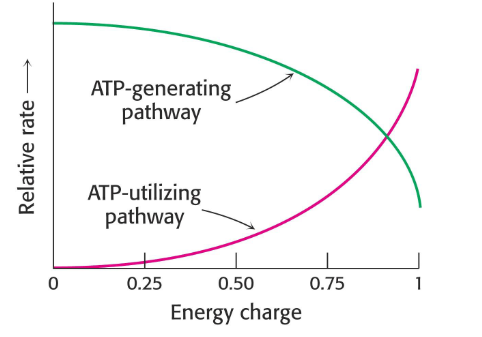
Energy Change
- a way to describe the energy status of a cell
- value can range from 0 (all AMP) to 1 (all ATP) in cell
- important in regulating some key metabolic enzymes
- we say 1 when ATP is predominant
- we say 0 when AMP is predominant