OCR A 3.1.3 Halogens
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
halogen elements colour
-grp 7
-F2: pale yellow gas
-Cl2: pale green gas
-Br2: brown/orange liquid
-I2: grey solid
grp 7 bp trend
-increases down grp
-london forces increase; due to increase in size and relative mass
grp 7 IE trend
-decreases down grp
-atom gets larger
-distance between +ve nucleus and bonding e increase
-more shielding
reactivity trend down grp 7
-decreases down grp
how to do grp 7 displacement reaction
-sol of each halogen added to aqueous sol oh another hallide
-eg: Cl2 + 2Br- →
-if halogen more reactive the halide present
-reaction will take place; sol changes colour
-to tell apart cyclohexane added and mixture is shook
why cyclohexane used
-halogen dissolve more readily in it
-helps to see colour change easily
-halogen present dissolves in organic layer which is above aqueous layer
Cl2 in water and cyclohexane
pale green
pale green
Br2 in water and cyclohexane
orange
orange
I2 in water and cyclohexane
brown
violet
Cl2 + KBr
-aqueous layer; orange
-organic layer; orange
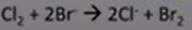
Cl2 + KI
-aq: brown
-org: violet
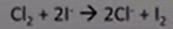
Br2 + KCl
aq: orange
org: orange
Br2 + KI
aq: brown
org: violet
I2 + KCl
aq: brown
org: violet
I2 + KBr
aq; brown
org: violet
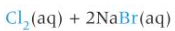
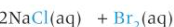
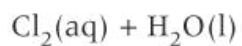
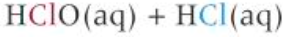
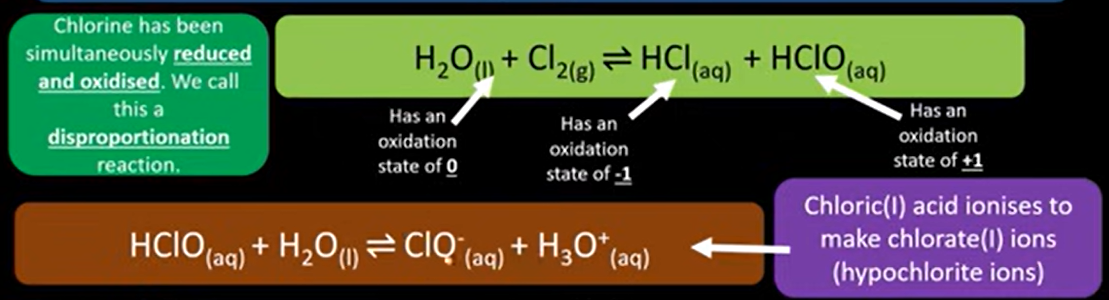
how to make bleach
-disproportionation reaction

uses of bleach
-treat water
-bleach fabrics/paper
-cleaning agent
how does Cl treat water
-when added to water; kills bacteria
-useful in drinking water and pools
Cl in drinking water advantages
-destroys microorganism that cause disease
-long lasting; reduces bacteria build up further down supply
-reduce growth of algae; that discolours water and gives bad taste and smell
Cl in drinking water disadvantages
-Cl gas is toxic; irritates respiratory system
-Cl liquid causes sever chemical burns to skin
-Cl can react w/ organic compounds in water to chloroalkanes; cause cancer
ethical issue with chlorinating water
-no choice if our water is chlorinated
-forced medication on population
alternatives to chlorinating water
ozone;
-powerful oxidising agent that kills microorganisms
-short half life
-non perm and expensive
UV light
-damages DNA in microorganisms
-not work in cloudy water
disproportionation
redox reaction which same element is simultaneously oxidised and reduced