Pain Medications and Anti-Arthritis Medications
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
Extra Notes
How Salonpas works is that the “heat” busies receptors and “distracts” them from the pain
If something is a factor it is generally a polypeptide
Pain and Classifications
complex sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage
even without pathological/tissue damage, it’s still pain
Can be classified based on:
Pathophysiology
Duration
Etiology
Location
Types of Pain Based on Pathophysiology
Nociceptive Pain: activation of nociceptors in response to noxious stimuli
ex: headache, back pain
involves inflammation
Neuropathic Pain: due to central sensitization or neuronal damage
ex: phantom pain, burning, tingling, numbing, sitting on hand, cancer, etc.
Nociplastic Pain:
changes in nociceptive pathway without evidence of nerve or tissue damage
due to oversensitization/Central Sensitization
ex: cancer pain; phantom pain (even if not there)
Mixed Pain: nociceptive and neuropathic origin
pain caused by mixture of various pathologies
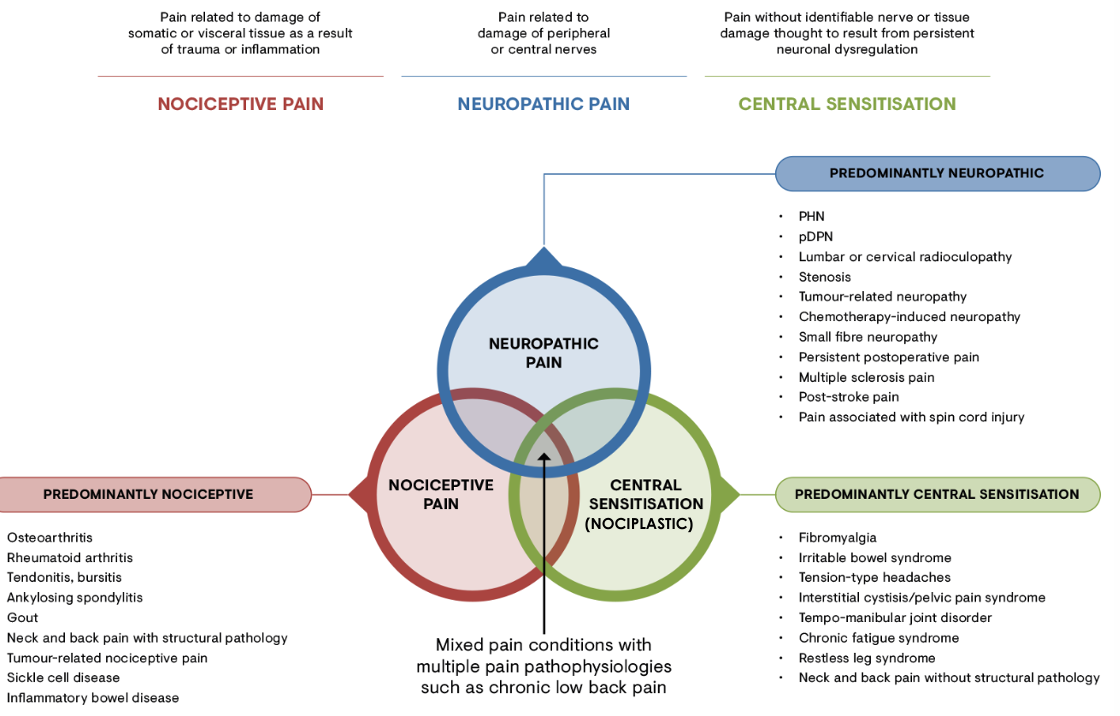
Peripheral Pain: can be Nociceptive or Neuropathic
Central Sensitization: can be Nociplastic or Mixed, some Neuropathic
Types of Pain Based on Duration
Acute Pain: immediate short term effect upon exposure to stimuli
few seconds to less than six months
Chronic Pain: sensitization at the level of spinal neurons via multiple mechanisms
can be episodal (not all the time, when triggered or seasonal, every night, etc.)
lasts for 6 or more months
Breakthrough Pain: pain in an already-treated patient due to movement, spontaneous or resulting from weaning off drugs or drug effects
ex: cancer pain, withdrawal
“breaks through” the current pain medication
Types of Pain Based on Etiology
Cancer Pain:
cause due to cancer itself, drug treatment for cancer, or associated disease
Chronic Non-Cancer Pain (CNCP):
may have multiple etiologies
ex: rheumatoid arthritis
Types of Pain Based on Location
Lower Back Pain:
ex: due to posture, strains, underlying disease, referred pain
Neck and Shoulder Pain:
due to strains, sprains, posture, spinal cord compression, injuries
Headaches:
due to posture, stress, migraines, underlying disease (ex: tumors)
Referred Pain:
visceral pain that radiates to surrounding region
pain is felt somewhere else other than the actual damaged part
Pain Pathways
Transduction
sensory nerve picks up noxious stimulus via nociceptor
pseudo-unipolar, single axon that branches to two (soma is to the side)
stimulus is picked up at the peripheral end
Transmission
signal is transmitted via peripheral axons (primary afferent fibers)
signal travels to somas in Dorsal Root Ganglion
Relay
central end axons of DRG neurons release chemical neurotransmitters
received by Spinal Cord Dorsal Horn Neurons (SCDH) (secondary neurons)
secondary neurons carry signal to Central Nervous System
also beginning point of Central Sensitization
Integration & Interpretation/Perception
signal is brought to higher brain and is processed
pain is actually perceived
Modulation
brain sends response signal through descending pathway
can either facilitate or inhibit pain
descending neurons synapse with Dorsal Horn, release neurotransmitters
ex: opioids, enkephalin, endorphins
Peripheral Sensitization up until Dorsal Root Ganglion (before Dorsal Column)
Central Sensitization starts in spinal cord
Drug that Target Specific Steps in Pain Pathway
Transduction:
Paracetamol
NSAIDs
Antihistamines
Opioids
Local Anesthetics
Transmission:
Opioids
Local Anesthetics
Integration & Interpretation:
Opioids
α2 Agonists
General Anesthetics
Modulation:
Opioids
α2 Agonists
NMDA Antagonists (N-methyl-D-Aspartate)
Peripheral Sensitization
due to inflammation
Chemokines
released by Neutrophils, Mast Cells, Macrophages, Vessels, etc.
ex: TNF-α, 5-HT, Histamine, Prostaglandins, Interleukins, etc.
or other noxious stimuli (ex: Capsaicin/Heat, acid, ATP, etc.)
bind to neuron, cause action potential (depolarization)
Potential spreads all the way to spinal neuron
Peripheral Receptors
Refer to note in previous card
Acid-Sensing Ion Channel:
senses protons
if too acidic, will signal for pain
P2X3:
ATP-Gated Ion Channel
Tyrosine Receptor Kinase B:
responds to BDNF (brain-derived neurotropic factor)
TRPV1:
responds to heat or capsaicin
Can be targeted by Paracetamol, NSAIDS, Antihistamines, Opioids, Local Anesthetics, Steroids
Central Sensitization
from peripheral nerve that reaches spinal neuron
leads to release of Substance P and BDNF (in pre-synapse)
have receptors in post-synapse in spinal column
Glial cells (brain) also release Interleukins and Tumor Necrosis Factor-α
Descending Pathway Neuron can also release glutamate
all receptors lead to release in Calcium
Calcium propagates signal until it reaches brain
Central Receptors
NMDA:
activated by glutamate
AMPA:
activated by glutamate
NK1:
Neurokinin-1
activated by Substance P
Tyrosine Receptor Kinase B:
activated by BDNF
Sodium and Calcium Channels:
allow for influx of cations
cause depolarization due to positive charge
also Ca-Channels increase intracellular calcium
Can be targeted by Paracetamol, Opioids, α2 Agonists, NMDA Antagonists
NSAIDS
Nonsteroidal Anti-Inflammatory Drugs
Pharmacophore:
generally have aromatic acid portion with other group attached
phenolic acid part confers activity
Types:
Salicylic Acid Derivatives
Aryl and Heteroaryl Acetic Acid Derivatives
generally Propionates
Indol and Indene Acetic Acid Derivatives
Anthranilic Acid Derivatives
Enolic Acid Derivatives
NSAIDS Mechanism
Arachidonic Acid:
Cell membrane contains C18 unsaturated fatty acid residues
phospholipase A2, removes phosphoglycerol head
triggered by various stimuli
converted into C20 arachidonic acid
main substrate for COX-I and COX-II, and 5-lipoxygenase
COX form prostaglandins
5-lipoxygenase forms leukotrienes
NSAIDS target COX-I, COX-II, and 5-lipooxygenase
prevent synthesis of pain-related eicosanoids
ideally selective for COX-II (inducible) and not COX-I (always expressed) (ex: celecoxib)
COX-I and COX-II Selective Binding
main substrate is arachidonic acid
COX-I is always expressed, maintains homeostasis
COX-II is only inducible
COX-II has a larger hydrophobic binding pocket
Valine residue instead of COX-I Isoleucine (one less C)
COX-II selective drugs have an extra protruding hydrophobic part to hook into that gap; and to prevent fitting into COX-I space
NSAIDS Toxicity
Due to effect over long-use
induced mitochondrial dysfunction and apoptosis
Intracerebral Hemorrhage
Aspirin decreases platelet aggregation, promotes microbleeding
Respiratory Complications
decreases PGI2 and increased inflammation (aspirin exacerbated respiratory disease)
Community Acquired Pneumonia - decrease in neutrophil recruitment due to chemokine decrease
Heart Injury
prevention of thrombosis (aspirin)
mitochondrial dysfunction, apoptosis
GI Mucosal Injury:
due to reactive prooxidants, apoptosis
Liver Injury
apoptosis, ROS
Kidneys
renal injury, hypertension, decreased water & sodium retention
Specific Mechanism:
NSAID Overdose → loosens tight junction of epithelial cells (due to acidity of drug)
makes paracellular transport easier
LPS (endotoxin) entry = inflammation
increase in toxic bile salt micelles = membrane disruption = apoptosis
disrupts proton gradient (due to acidity/H+ flowing back in) = decreases ATP Production
picks up proton in matrix, releases it in cell
increased ROS = DNA damage
Paracetamol
also acetaminophen (acetyl amine + phenol)
technically not anti-inflammatory
analgesic (less effective than NSAIDs), antipyretic
targets COX-3 which is more in Central Nervous System
is both central and peripheral acting (more central; can exist as molecular form, not charged = permeation)
main metabolite is aminophenol, which is also central acting
can be used for acute and chronic pain (but not as effective)
maximum dose: 4g/day
recommended dose: 500-1000 mg; q 4-6 hours
Paracetamol MOA
targets COX-1 protein variant in central
targets COX-2 in central
COX1/2 inhibition leads to antipyretic effect
can also activate TRPV1 and T-Type Calcium Channels
might lead to analgesic effect
Paracetamol Toxicity
can occur even at regular doses, also occurs as toxic doses
Regular Dose Toxicity Examples:
GI Complaints
Hypersensitivity, Angioedema (swelling)
Kidney damage
GI Bleeding (doses taken >2g/day continuously)
Stevens-Johnson Syndrome & Toxic Epidermal Necrolysis
Agranulocytosis, Anemia, Thrombocytopenia
Small increased Systolic BP, hypertension
Toxic Dose Toxicity Examples:
serious liver damage, acute liver failure
nausea, vomiting, sweating
pain in right hypochondrium (due to liver)
increase in aminotransferases (due to liver damage releasing)
acute kidney injury
skeletal muscle cytosis
hepatic encephalopathy (brain problem due to liver problem)
Paracetamol Metabolism and Toxicity
95% is metabolized by conversion to glucuronide sulfates (Phase II) → excretion in urine
5% is converted to NAPQI (Quinone Intermediate) via Phase I
intermediate is toxic
Rescued by Glutathione, conjugates to form non-toxic product, excreted in urine
but those that it misses can cause hepatoxicity (due to electrophilicity)
ex: attacked by SH of cysteine residues
Regular Continuous Consumption = run out of glutathione = NAPQI will cause hepatoxicity
also because so much is taken, that 5% becomes a lot
Opioid Analgesics
can be natural, semi-synthetics, and fully synthetic
can hit almost all steps in pain pathway
Endogenous: Endorphins and Enkephalin
Mechanism:
inhibition of presynaptic neuron release (ex: Substance P) due to preventing Calcium influx
activation of opioid receptor
are inhibitory G-Protein Coupled Receptors
lowers intracellular calcium in pre-synaptic neuron
prevents cAMP as well by inhibiting adenylyl cyclase
associated receptor with analgesia: MOR (μ-opioid receptor
promotes K-Channel, inhibits Ca-Channel, prevents action potential propagation/depolarization
Opioid Pharmacophore
due to similarity to enkephalin
side from OH to NH portion
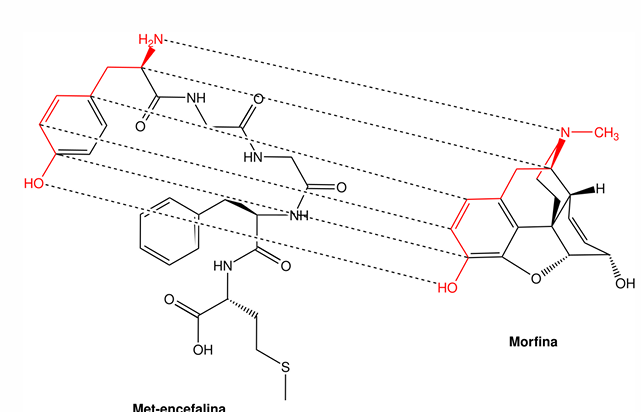
μ-opioid receptor
Inactivated State:
closed state
G protein is not activated, so no overall inhibition of process
state promoted by antagonists
Activated State:
open state
activates G protein, causes inhibitory effect
state promoted by agonists
Opioid Toxicity
Common Effects:
constipation
nausea, vomiting
sedation
pruritus (itch)
Less Common Effects:
dry mouth
urinary retention
respiratory depression
mental confusion
Tramadol:
also has somnolence (drowsiness)
Adjuvant Analgesics
agents not initially meant for pain, but also do inhibit pain
ex: Antiepileptic, Tricyclic Antidepressants, SNRIs, etc.
aid in the slowing down of pain, or affect perception of pain
Antiepileptic Agents (GABA Receptors Agonists)
ex: gabapentin
GABA Receptors are also inhibitory G-Protein receptors
open chloride channels, allow chloride to flow in
causes cell hyperpolarization, prevents depolarization for signal transduction
causes inhibitory effect
sedation, analgesic effect
Kohane VA Struck by MizuEna Beams

Arthritis Types
Rheumatoid Arthritis
thinned cartilage
synovial area is highly inflamed
immune system attacks joints
Osteoarthritis
shortened cartilage, no synovial insulation
bone ends rub together, causing pain
DMARDs do not work (due to not being caused by inflammation)
Gouty Arthritis
due to monosodium urate crystal buildup (from uric acid)
unable to be excreted, builds up at joints
crystals cause inflammation and pain
Rheumatoid Arthritis Pathophysiology
unknown trigger promotes inflammation in synovial membrane
attracts leukocytes
Autoreactive CD4 T Cells activate macrophages → pro-inflammatory chemokine production
Chemokines induce MMPs and RANK ligand production by fibroblasts
RANK ligand activates osteoclast and MMP destroys tissue
Treatment: prevention of release/production of chemokines
Antirheumatoid Drugs
DMARDs (Disease Modifying Anti-Rheumatoid Drugs)
split into biological
TNF-a inhibitors
IL-1 antagonists
generally monoclonal antibodies (bind the chemokines)
and nonbiological
immunosuppressant
Adjuvant Drugs
Methotrexate (MTX) and Sulfasalazine
MTX:
originally anticancer agent
enters cell as prodrug
converted into MTX-PG (added glutamate)
folic acid analog
affects DNA synthesis (decreases thymine)
but for this pathway, inhibits ATIC enzyme
causes buildup of AICAR
inhibits Adenosine Deaminase, leads to increased adenosine
which is exported and binds to extracellular receptors, causing anti-inflammatory effects
Sulfasalazine:
inhibits AICAR conversion to IMP
this increases adenosine and also increases AICAR
AICAR may promote anti-inflammatory response
Note: inhibited DNA synthesis also prevents rapid cell proliferation (which limits immune cells)
Longer effect; but does end up modifying disease (as opposed to just symptom inhibition)
Gold Compounds
contain gold
work by inhibiting thioredoxin reductase
prevents DNA replication which prevents leukocyte attack, and eventually, ends inflammation
BIBIBIBIBIBIBI

Drug Targets for Gout
can either attack:
Acute Gout Attack:
increase in uric acid and decrease in PH → crystal deposits forming
Purine Metabolism:
Uric acid a product of purine breakdown
can inhibit xanthine oxidase (prevents formation of xanthine and uric acid)
can also inhibit reabsorption
Can be treated by:
NSAIDs
Corticosteroids
Colchicine (caution in kidney disease, may cause bone marrow suppression)