Intermolecular Forces and Properties of Solutions in Chemistry
1/171
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
172 Terms
Intermolecular Forces (IMF)
Attractive forces that molecules exert on one another, with strength related to the distance between particles.
Kinetic Energy (KEavg)
The average kinetic energy of molecules, which increases with temperature.
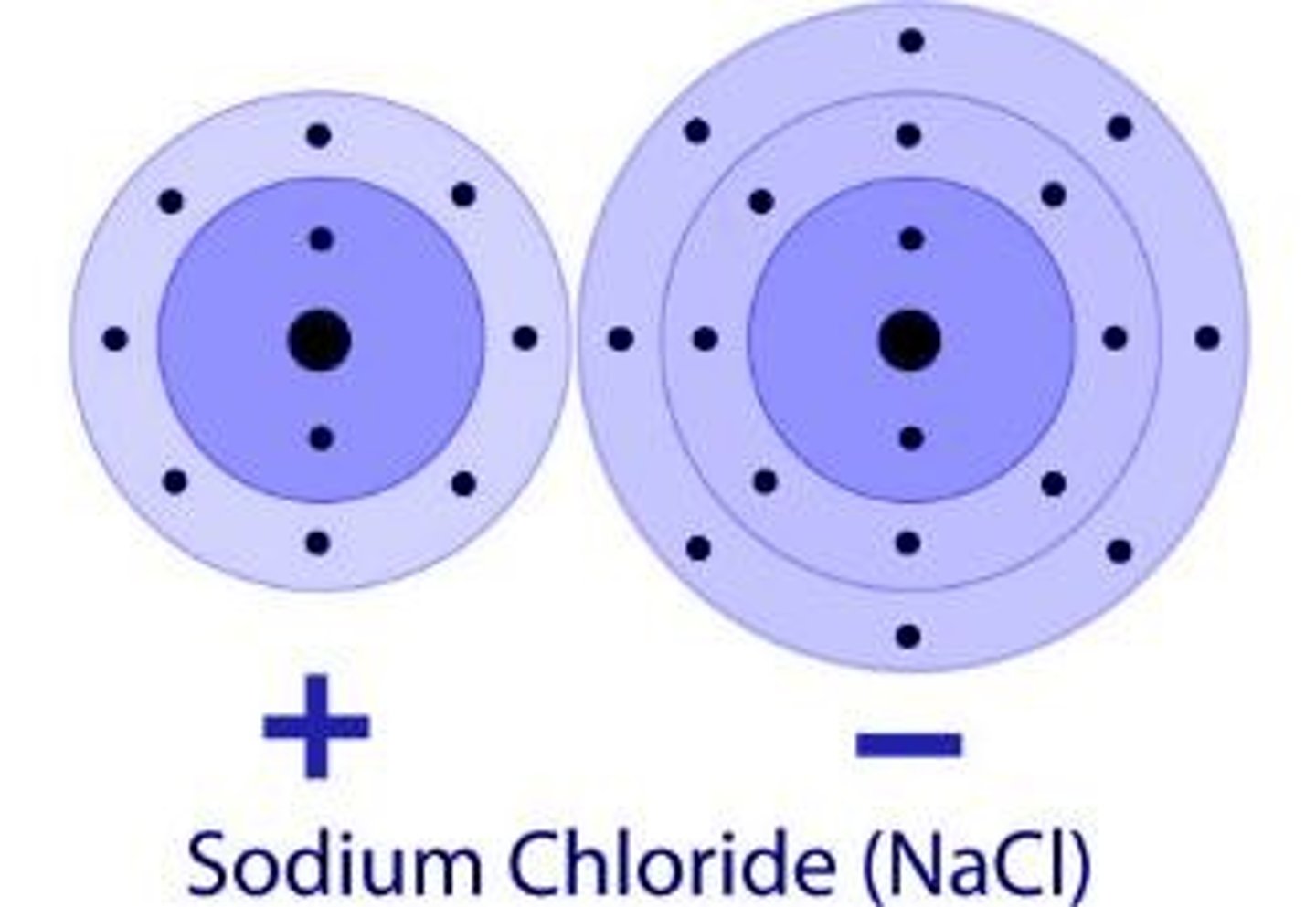
Ionic Bond
A bond formed when an atom donates electrons to another atom, typically between a nonmetal (anion) and a metal (cation).
Covalent Bond
A bond formed when two or more atoms share electrons, typically between nonmetals.
Metallic Bond
A bond formed between metal atoms, characterized by a sea of shared electrons.
Nonpolar Molecule
A molecule with an electronegativity difference of 0-0.4, where electrons are equally distributed.
Polar Molecule
A molecule with an electronegativity difference of 0.5-2.0, characterized by unequal distribution of electrons.
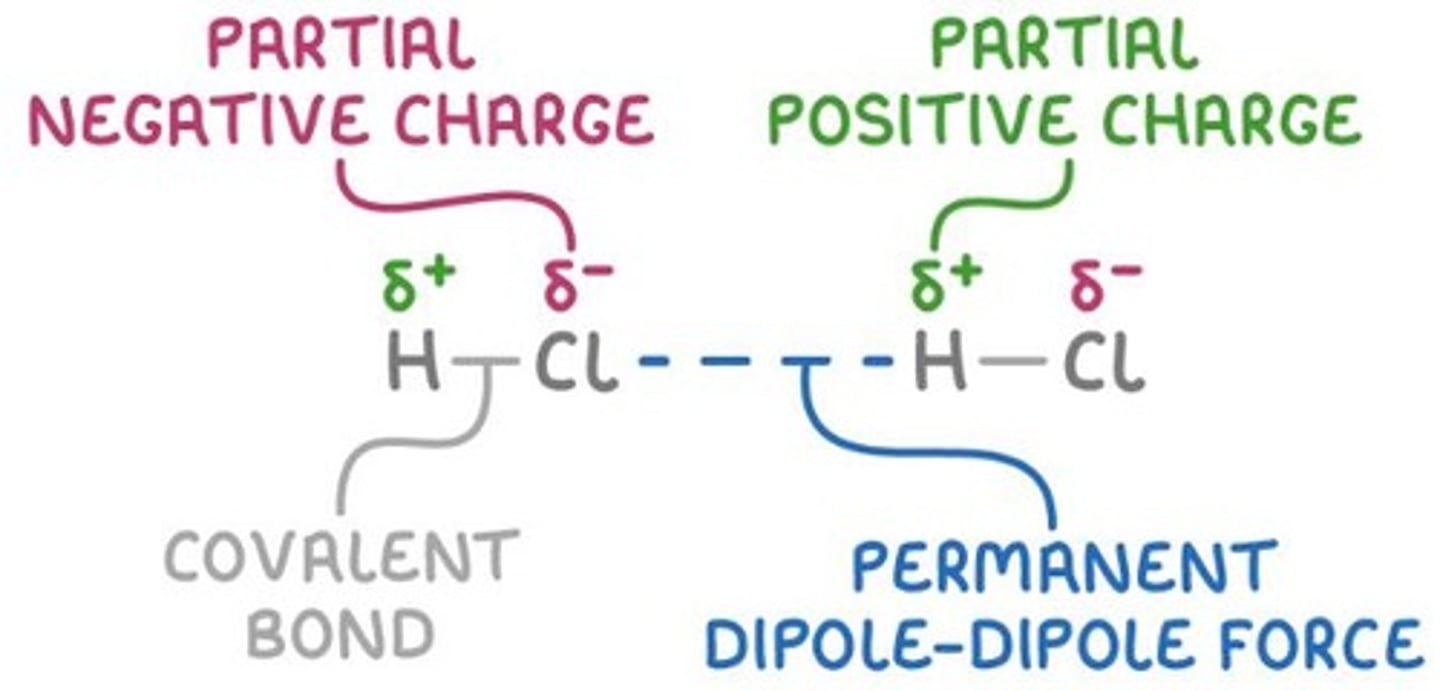
Dipole-dipole Interaction
An intermolecular force present in polar molecules.
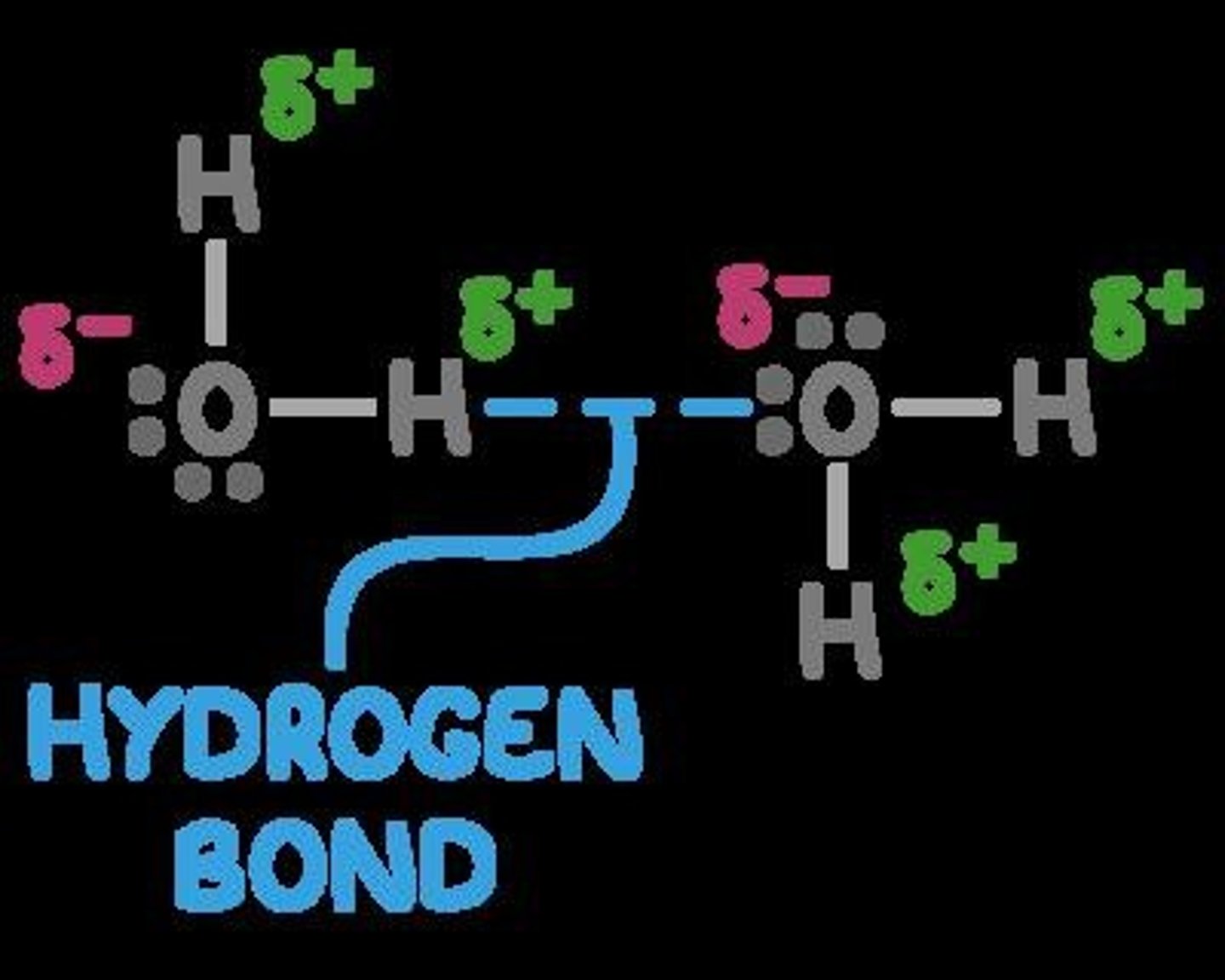
Hydrogen Bonding (H-bond)
A strong intermolecular force present in polar molecules with hydrogen atoms bonded to fluorine, oxygen, or nitrogen.
London Dispersion Forces (LDF)
Weak intermolecular forces that exist in all molecules, more significant in nonpolar molecules.
Ion-ion Interaction
Interactions between ions in ionic compounds.
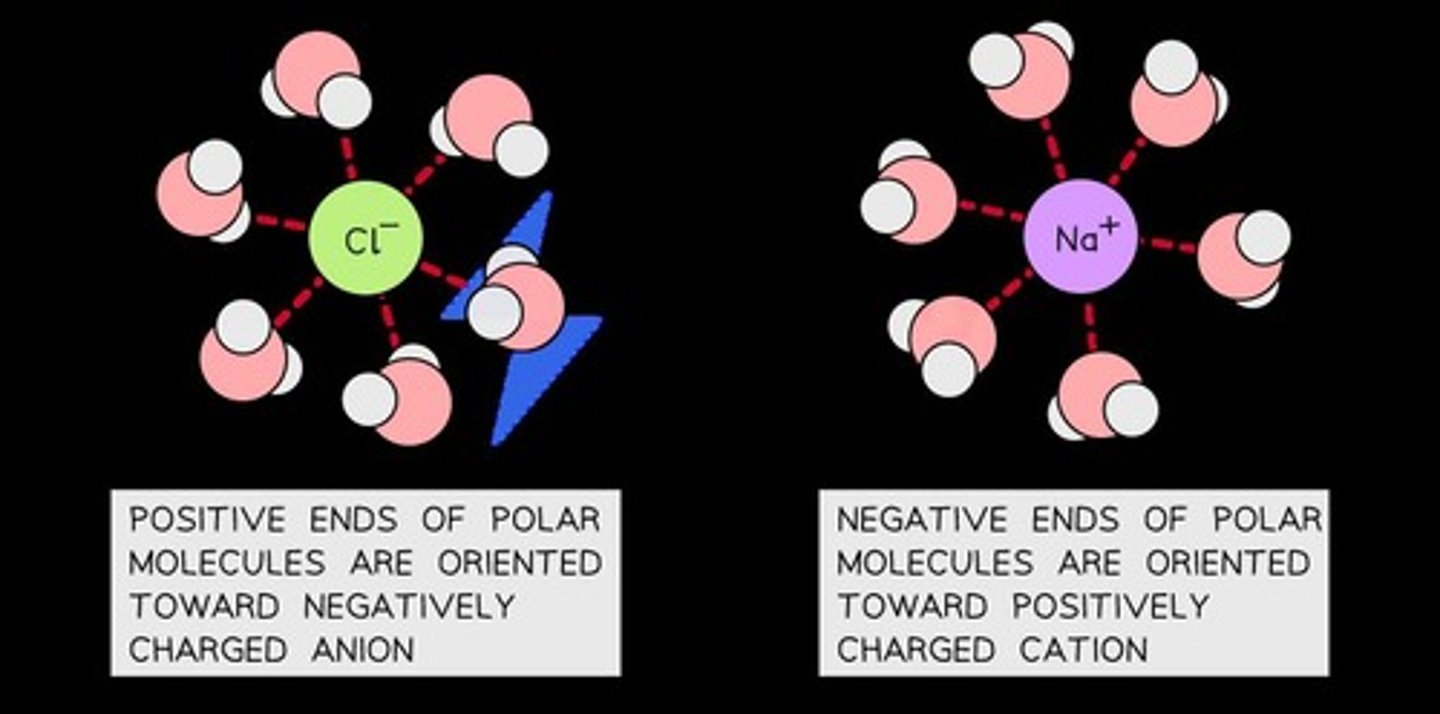
Ion-dipole Interaction
An interaction between ion/ionic compounds and polar molecules.
Induced-dipole Interaction
Occurs between nonpolar molecules and ions/polar molecules when a temporary dipole is induced.
Strength of IMF
The hierarchy of intermolecular forces from strongest to weakest: Ion-ion, Ion-dipole, H-Bonding, Dipole-dipole, LDF.
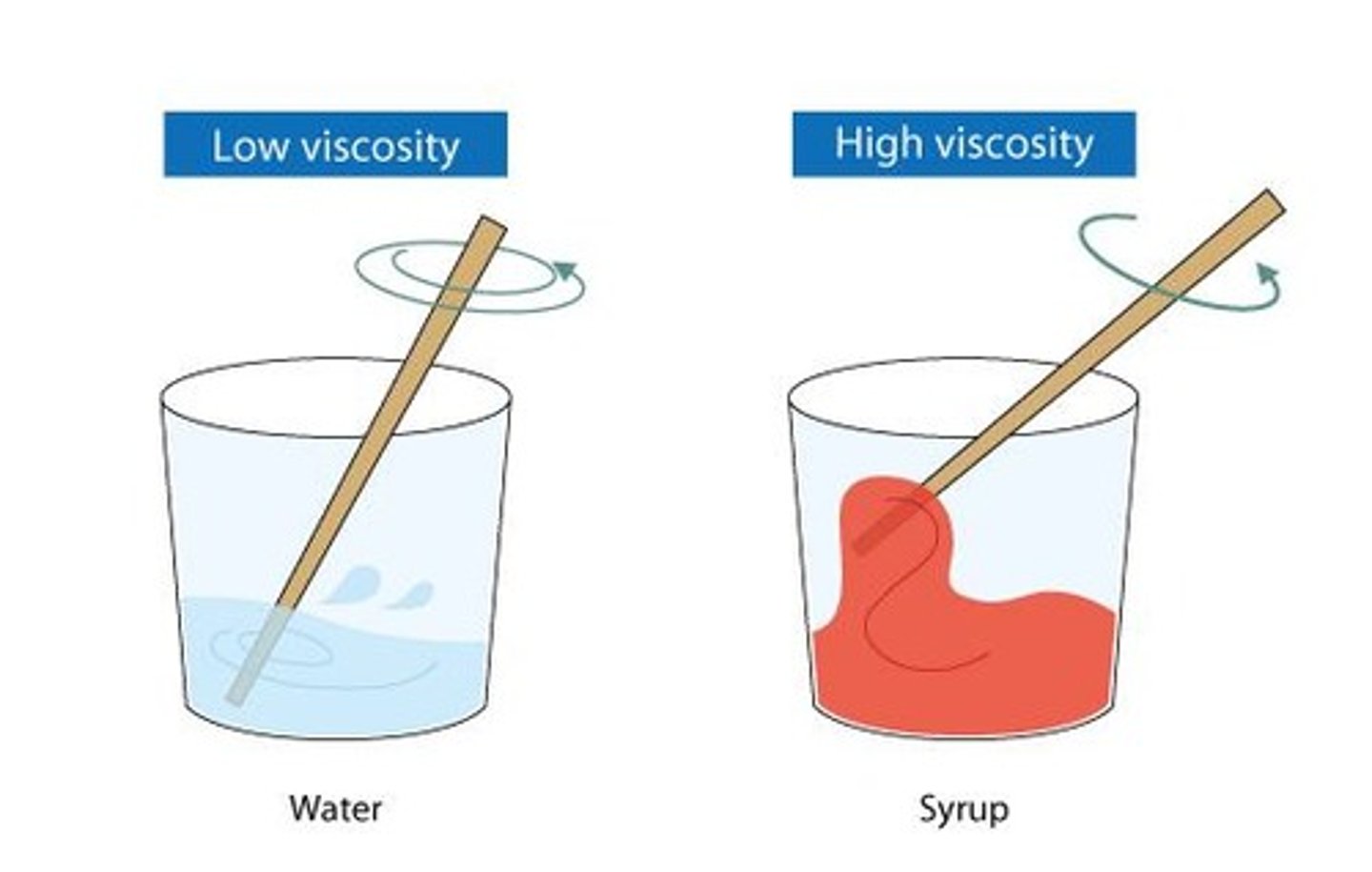
Viscosity
A measure of a fluid's resistance to flow; influenced by intermolecular forces and temperature.
Boiling Point
The temperature at which a liquid's vapor pressure equals the external pressure.
Melting Point
The temperature at which a solid becomes a liquid.
Vapor Pressure
The pressure exerted by a vapor in equilibrium with its liquid or solid form.
Stronger IMF
Results in higher boiling and melting points, and lower vapor pressure.
Weaker IMF
Results in lower boiling and melting points, and higher vapor pressure.
Viscosity
Measure of a fluid's resistance to flow; liquids that flow fast have lower viscosity and liquids that flow slow have higher viscosity.
Intermolecular Forces (IMFs)
Forces that help liquids take up space in a container and are directly related to surface tension, viscosity, and vapor pressure.
Surface Tension
The energy required to increase the surface area of a liquid; higher in liquids that have higher intermolecular forces.
Surfactants
Substances that reduce surface tension between liquids and solids.
Cohesion
The binding of like molecules.
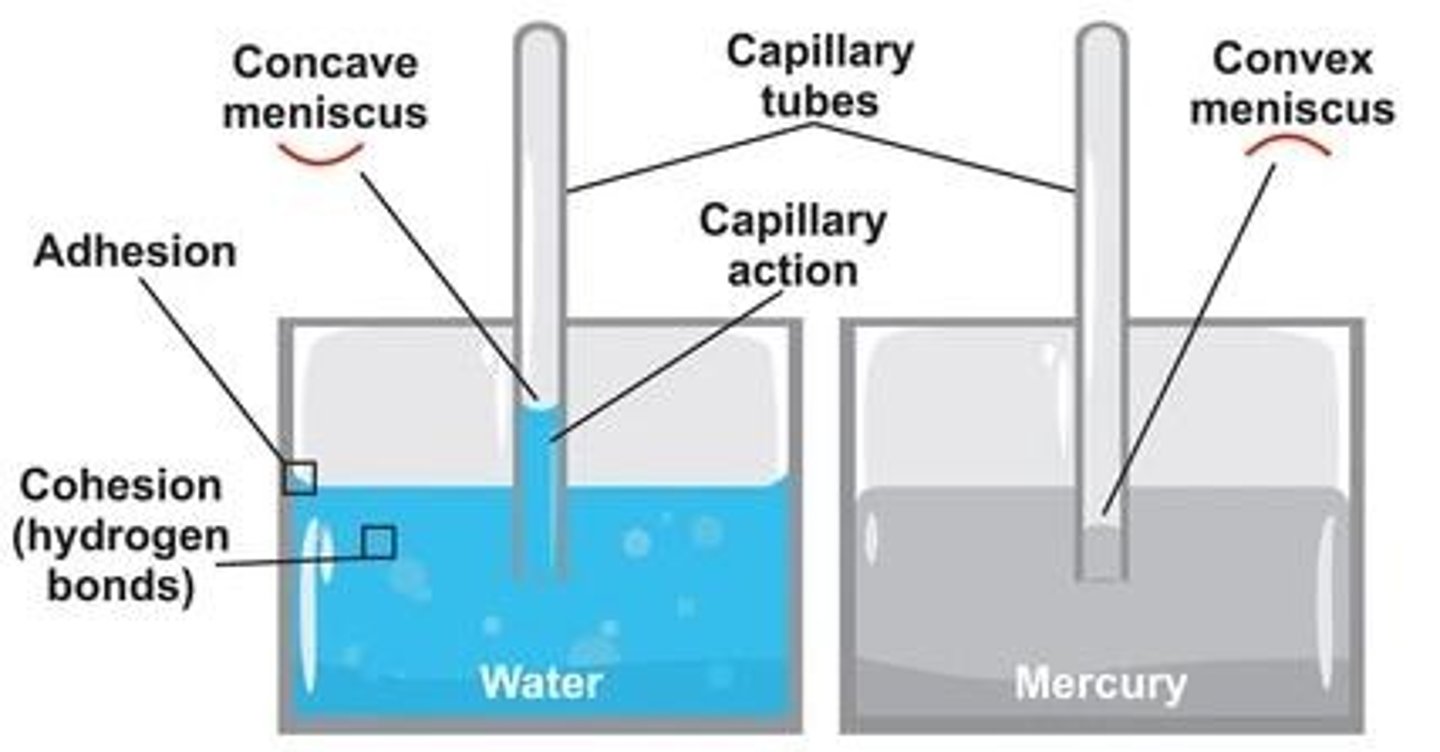
Adhesion
The binding of a substance to a surface.
Meniscus
Curved surface of a liquid.
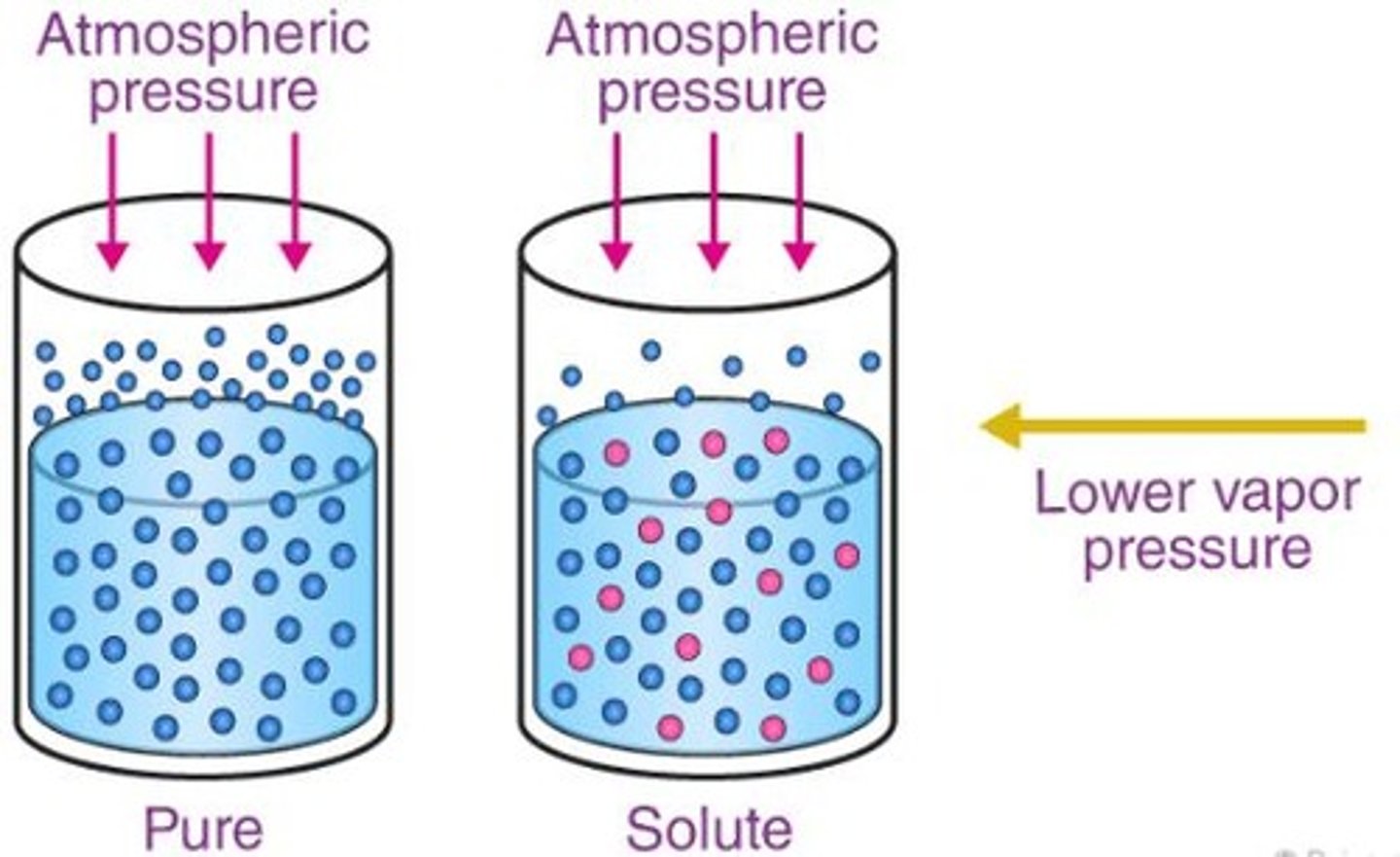
Vapor Pressure
Pressure exerted by vapor equilibrium with its liquid phase in a closed system.
Solubility
Ability of a substance to dissolve a given amount of solvent at a specified temperature.
Miscible Liquids
Liquids that are able to mix.
Immiscible Liquids
Liquids that are unable to mix.
Hydrophilic
Substances that can interact with water.
Hydrophobic
Substances that repel water.
Amphipathic Molecules
Molecules that have both hydrophilic and hydrophobic regions.
Properties of Water
Represented by the chemical formula H2O; known to be the universal solvent due to its capability to dissolve most substances.
Crystalline Solids
Solids arranged in a definite repeating pattern, held by a uniform strong IMF.
Amorphous Solids
Solids that lack the order found in crystalline solids and have structures at the atomic level similar to liquids.
Unit Cell
The small repeating unit in the structure of crystalline solids.
Crystal Lattice
The arrangement of geometrical patterns of points in a unit cell.
Motif
An atom or a group of atoms that is repeated at each lattice point to generate a crystal structure.
Metallic Solids
Solids that consist entirely of metal atoms, characterized by high electrical and thermal conductivity.
Heat Capacity
The amount of heat required to raise the temperature of a substance by 1°C.
Specific Heat Capacity
The amount of heat required to raise the temperature of 1g of a substance by 1°C.
Melting Point
The temperature at which a solid becomes a liquid; for water, it is 0°C.
Boiling Point
The temperature at which a liquid becomes a gas; for water, it is 100°C.
Covalent Network Solids
held together by covalent solids
Properties of Covalent Network Solids
poor conductors, high melting points, has allotropy, characterized by strength or hardness
Allotropy
the ability of a single element, atom, or molecule to form multiple structures of solids
Molecular Solids
made up of covalently bonded atoms that are held together by IMFs such as Van der Waals or weak dispersion forces
Properties of Molecular Solids
poor conductors (insulators), soft, high melting point, easily dissolve in water
Ionic Solids
composed of cations and anions that are held together by electrostatic attractions
Properties of Ionic Solids
difficult to break, high melting point, poor conductors, electrostatic attractions are stronger than Van der Waals
Melting Point
the temperature at which a solid loses definite shape and is converted to liquid
Freezing Point
the temperature at which a liquid changes to a solid
Heat of Fusion
the quantity of heat necessary to melt a solid
Sublimation
solid to gas
Deposition
gas to solid
Enthalpy of Sublimation
the quantity of heat to convert solid to vapor
Malleability
ability of a solid to undergo compressive stress without breaking it
Ductility
the ability of a solid to undergo tensile stress or stretched without fracture
Electrical Conductivity
the measurement of the ability of atoms, molecules, or ions to transfer electrons from one to another
Thermal Conductivity
the measurement of the ability of atoms, molecules, or ions to move and collide with its neighboring particles
Solid Solutions
homogeneous mixtures that appear as one phase, composed of solute particles dissolved in an appropriate solvent
Solute
substance being dissolved
Solvent
dissolving the substance
Unsaturated Solution
when the amount of solute is less than the solute's solubility at a given volume and temperature
Saturated Solution
when the amount of solute is equal to the solute's solubility at a given volume and temperature
Solubility
the ability of a solid, liquid, or gaseous chemical substance to dissolve in a solvent and form a solution.
Supersaturated Solution
when the amount of solute is greater than the solute's solubility at a given volume and temperature
Nature of Solute and Solvent
"like dissolves like" - solute and solvent molecules with the same IMFs are soluble to one another
Temperature
the most evident factor that affects solubility; most solids are soluble at higher temperatures; solubility of gases decreases as temperature increases.
Concentrated Solution
when a solution contains an excessively large amount of solute; molarities > 1M
Diluted Solution
solution of low concentration; molarities < 1M; prepared through the process of dilution
Percentage by Mass (%m/m)
𝑚=𝑚𝑎𝑠𝑠 𝑠𝑜𝑙𝑢𝑡𝑒/𝑚𝑎𝑠𝑠 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛× 100
Percentage by Volume (%v/v)
% 𝑚=𝑣𝑜𝑙𝑢𝑚𝑒 𝑠𝑜𝑙𝑢𝑡𝑒/𝑣𝑜𝑙𝑢𝑚𝑒 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛× 100
Percentage by Mass/Volume (%m/v)
% 𝑚=𝑚𝑎𝑠𝑠 𝑠𝑜𝑙𝑢𝑡𝑒/𝑣𝑜𝑙𝑢𝑚𝑒 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛× 100
Pressure or Volume
does not significantly affect the solubility of solids and liquids but greatly affects the solubility of gases; the greater the pressure the greater the gas dissolves in a solution
Henry's Law
the relationship between pressure of the gas over the solution and solubility of the gas; 𝑐𝑔𝑎𝑠=𝑘𝐻𝑃
Concentration of gas (M)
𝑐𝑔𝑎𝑠
Pressure of gas (atm)
𝑃
Henry's constant (M/atm)
𝑘𝐻
mass solution
mass solvent + mass solute
volume solution
volume solvent + volume solute
mole solution
mole solvent + mole solute
%volume
proof no. / 2
density
mass/volume
Molarity (M)
M= mol solute / L solution
Molality (m)
m= mol solute / kg solvent
Mole (n or mol)
n = mass (m) / molar mass (M)
Mole Fraction (𝜓)
𝜓= mol solute or mol solvent / mol solution
Parts per Million (ppm)
ppm= mass solute / mass solution × 10^9
Parts per Billion (ppb)
ppb= mass solute / mass solution × 10^12
Raoult's Law
The vapor pressure of an ideal solution (P) is the product of the pure solvent (Po) and the mole fraction of the solvent (XB) in the solution at the given temperature.
Colligative Properties
Properties of solutions that are dependent on the concentration of solute particles and not on the nature or mass of solute.
ΔP
ΔP = vapor pressure lowering
Boiling Point Elevation
The addition of solute in a solvent results in increased boiling point.
Boiling Point Elevation Formula
Tb = To + ΔTb
ΔTb
ΔTb = m * kb
Vapor-Pressure Lowering
Depends on the number of solute particles that have been added in the solution.
Volatile Substance
A substance that evaporates quickly and exhibits vapor pressure.