Models of Atomic Theory - Scientists & Models
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
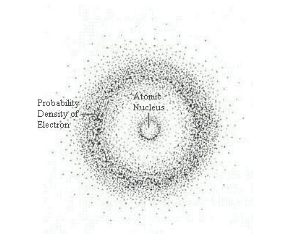
Who developed the Electron Cloud Model of Atomic Theory?
Erwin Schrodinger

Who developed the Sphere Model of Atomic Theory?
John Dalton
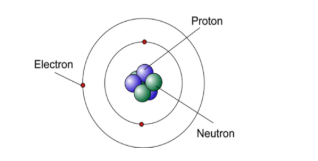
Who developed the model of atomic theory pictured below that included both protons and NEUTRONS in the nucleus of an atom?
James Chadwick
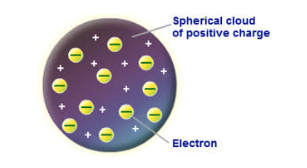
Who developed the Plum Pudding Model of Atomic Theory?
JJ Thompson
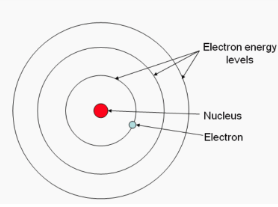
Who developed the model of atomic theory pictured below that included both protons and NEUTRONS in the nucleus of an atom?
Neils Bohr
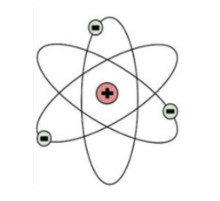
Who developed this model of atomic theory pictured below in which there was the recently discovered nucleus only containing a positively charged proton at the time and electrons revolving around it? This person also utilized the Gold Foil Experiment to develop this model.
Earnest Rutherford