Moles Relations
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
List 3 examples of counting units
pair of dice is two die
a dozen egg is 12
bale of paper is 5000 sheets
Define mole
unit used when counting atoms or molecules
What does a mole represent
6.022×1023 of entity counted (particles in the case of atoms or molecules)
Define molar mass
amount of mass present in 1 mole of a substance
What is molar mass of an element equal to
mass of 1 mol of atoms of that element
Explain how molar mass of an element and atomic weight are related
a mole of an element has a mass in grams equal to its atomic weight in amu
What can we use Avogadro’s number and molar mass to convert between
units of mass and particle number
What is the formula to convert moles to mass
multiple by molar mass
What is the formula to convert mass to mole
divide by molar mass
How to convert from mol to number
multiply by Avogadro’s number
How to convert from number to mole
dividing by Avogadro’s number
Explain whether we can convert mass directly to number
no, we have to convert mass to moles 1st
What does molecular formula provide
a mole ratio of atoms in a compound/unit
Water (H2O) has how many atoms
2 H atoms, 1 O atom
1 mole H2O has how many moles
Two moles H atoms, 1 mole O atoms
How is molar mass found (3 things)
identifying the atoms in a molecule
how many of each atom
molar atomic mass of each atom
What does each element contribute to
a fraction of the compound’s mass
How to determine mass % for a molecule of compound
molecular mass and chemical formula
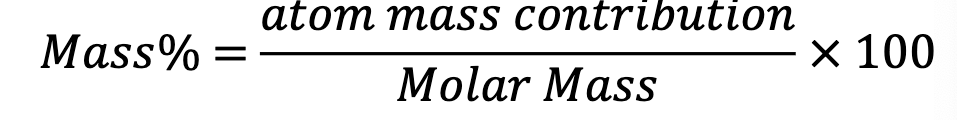
How to find mass % on a mole basis for a mole of compound
molar mass and formula