Chemical calculations
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
How is the relative atomic mass of an element found?
↳ Number at the top
↳ Usually higher
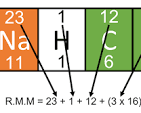
How do you work out relative atomic mass (Mr)?
↳ Add al the relative atomic masses together (top number)
What is Avogrado’s constant?
↳ One mole of substance contains
6.02 × 1023 atoms,ions or molecules
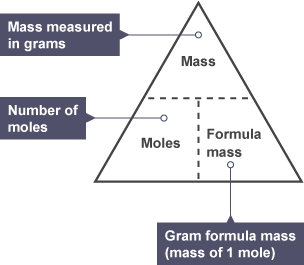
How do you work out moles?
↳ Mass ÷ Mr (add top numbers)
How do you work out concentration?
↳ Mass ÷ volume
How do you convert cm3 to dm3
↳ Divide by 1000
How do you work out the mass of product formed given the mass of a reactant?
↳ Balance the equation
↳ Work out moles (Mass ÷ Mr)
↳ Mass= moles x Mr (add top numbers)
How do you balance an equation given the masses of reactants and products?
↳ Work out Mr (add top numbers)
↳ Work out moles (Mass ÷ Mr)
↳ Convert to the simplest whole number ratio
↳ Balance symbol equation
How do you work out which reactants are in excess?
↳ Balance the equation
↳ Work out moles
↳ Compare moles
↳ Lower- limiting
↳ Higher- excess