Unit 1.2 Nucleic Acids/Properties of Water Quiz Review
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
What is deoxyribonucleic acid
DNA
What is ribonucleic acid
RNA
What are the 3 components of a nucleotide
A sugar with 5 carbon (pentose sugar), An acid phosphate group (negatively charged), and one of the 5 bases that contain nitrogen
Which of the 5 bases can’t be in RNA. Cytosine, Uracil, Thymine, Guanine or Adenine
Thymine
Which of the 5 bases can’t be in DNA. Cytosine, Uracil, Thymine, Guanine or Adenine
Uracil
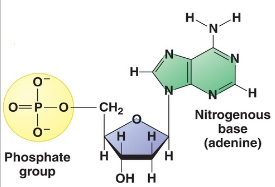
Is this DNA or RNA
DNA
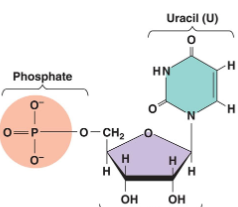
Is this DNA or RNA
RNA
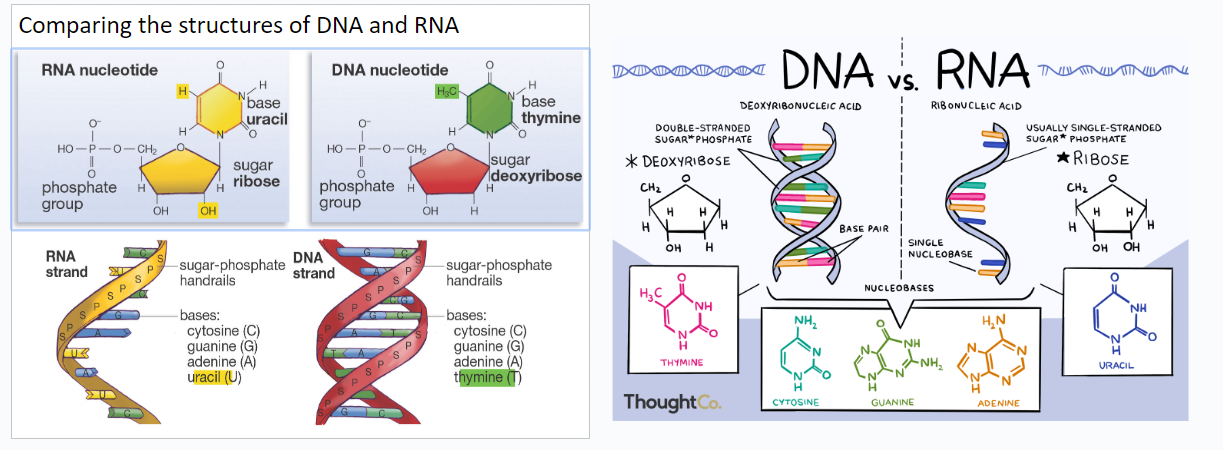
What makes the difference between DNA and RNA
RNA contains 2 OH at the bottom, DNA contains one OH and H at the bottom. uracil only contains an H on the top left while Thymine contains H2C
How are nucleotides linked together to polymers in long chains
Through condensation to form covalent bonds through a “sugar-phosphate backbone”
What are the strands that form a double helix called
Antiparallel
What does Adenine always pair with?
Thymine (DNA) or Uracil (RNA)
What does Guanine always pair with?
Cytosine
Why is complementary base pairing important
Keeps regular arrangement and geometry, also lets exact copies be made during replication.
Where are hydrogen bonds formed in DNA
The electrostatic force of attraction between hydrogen with an electronegative atom which is either (N, O, or F)
How many strands does RNA have
1
How many strands does DNA have
2
Where is DNA found in a cell
Nucleus
Where is RNA found in a cell
Cytoplasm
What type of molecules are they
Nucleic acid
What is the job of DNA and RNA
To store & express genetic info
Which part of storing and expressing genetic info does RNA do?
Express
Which part of storing and expressing genetic info does DNA do?
Store
What’s the difference in base pair rule between DNA and RNA
In RNA instead of Adenine and Thymine it’s Adenine and Uracil.
What are hydrogen bonds
The bond(s) between multiple molecules where hydrogen bonds to either (Oxygen, Nitrogen, or Fluorine)
What are the bonds within a molecule
Covalent bonds are the bonds that make a molecule
What is the difference in thermal conductivity of air and water
Air had less thermal conductivity because they’re spaced out and bump less. Water molecules constantly bump into each other and conduct heat more.
Why does water’s high thermal conductivity make it a good coolant?
When the water conducts heat it, it also removes it so when you put it on a heated surface the water heats up and the surface cools.
Why does water have a high heat capacity
It takes lots of heat to heat up water because most of the heat goes into breaking hydrogen bonds between water molecules, so it requires lots of heat to break these bonds and heat up the water.
What is capillary action?
It’s both cohesion and adhesion. Adhesion allows water to bond to other surfaces and molecules (outside of water molecules). Cohesion is the bond between other water moles.
How does buoyancy work in water?
The bond between water molecules through cohesion let certain things float. As long as the object density is less than the fluid density. When it’s equal or less it will float. By dispersing weight you apply less pressure in one area which prevents water molecules from breaking.
What is the difference of adhesion and cohesion.
Adhesion is between water and other molecules
(different molecules)
Cohesion is between water and water molecules (same molecules)
What is viscosity
The resistance of flow, so air has little to no viscosity which is why we can easy walk through it. Water is a little higher but still low in viscosity compared to something like honey. It takes to go through water but MORE to go through honey. So honey has high viscosity and water has low viscosity.