covalent bonding in water, ammonia and methane
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
examples molecules containing several atoms
water
ammonia
methane
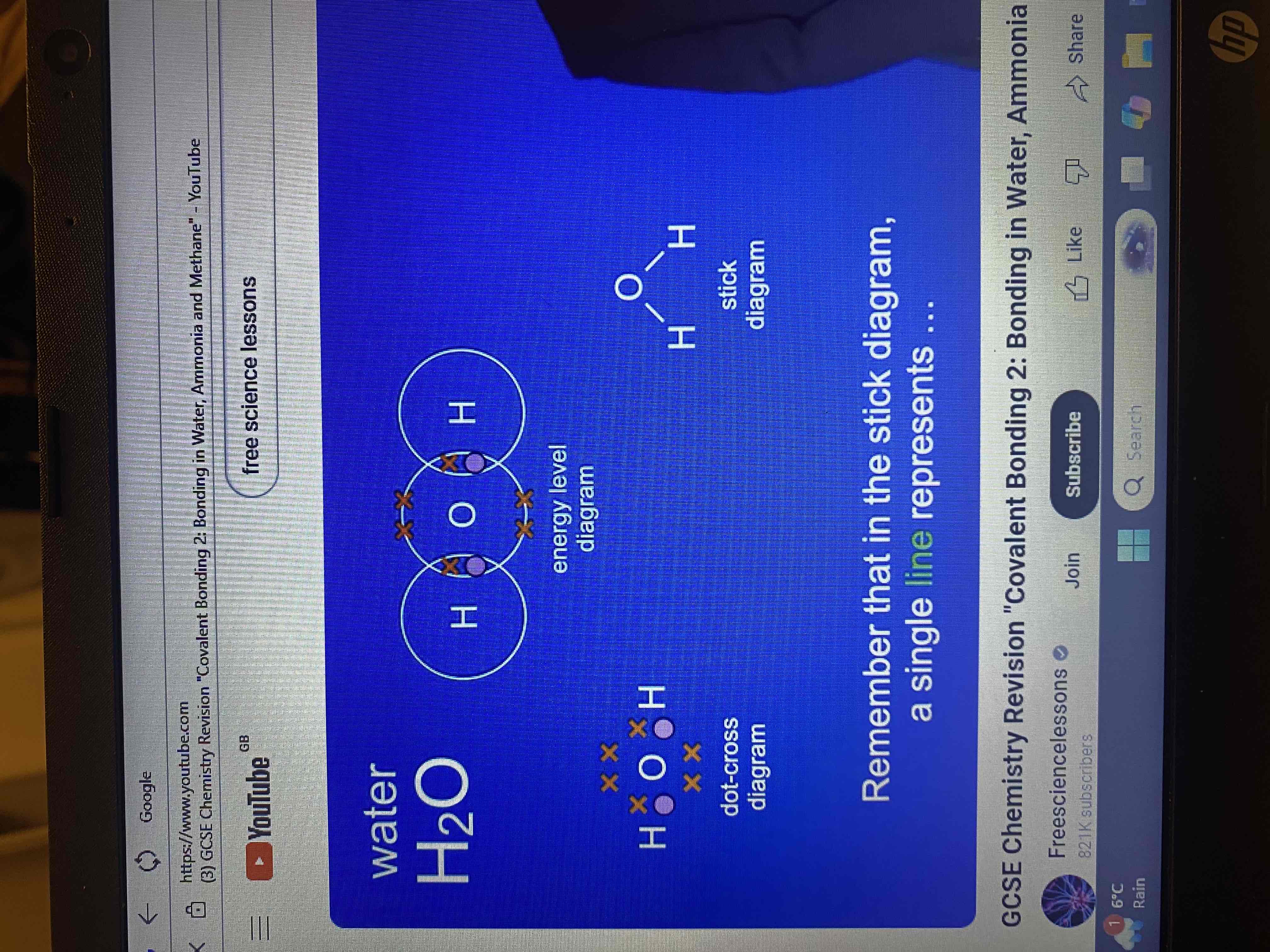
what does the formula for water (H₂O) tell us?
that a water molecule contains two atoms of hydrogen and one atom of oxygen.
oxygen has 8 electrons how many electrons are in the first energy level?
two electrons
how many electrons are there in the outer energy level?
six electrons
both hydrogen atoms require how many electrons to achieve a full outer energy level?
one electron
how many electrons does the oxygen atom require?
two more electrons
how can we achieve this?
by overlapping the outer energy levels for the hydrogen atoms and oxygen atoms.
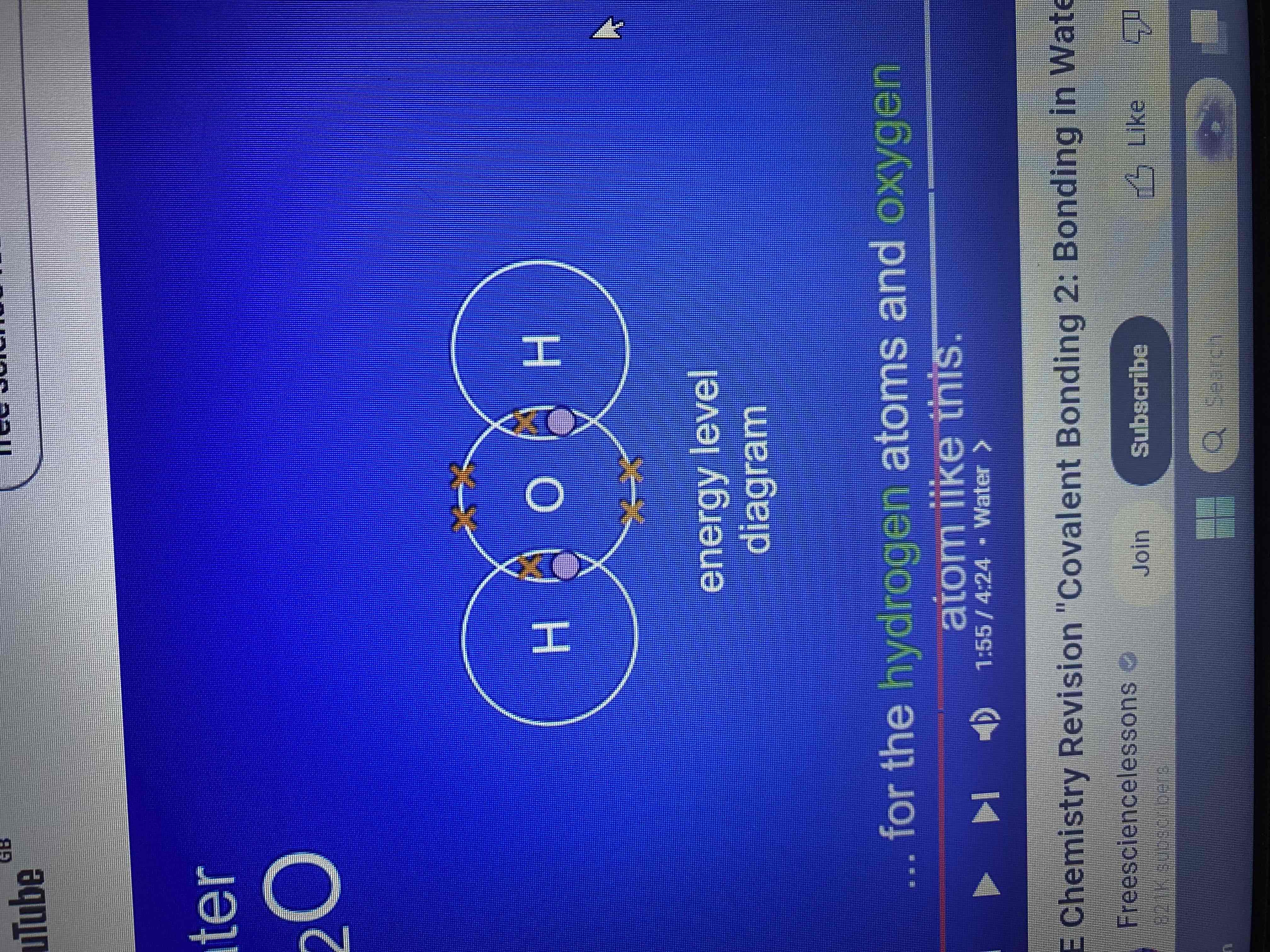
by sharing electrons what do both the hydrogen atoms and oxygen atoms have?
a full outer energy level
what is shared pair of electrons?
a covalent bond
how many covalent bonds does the water molecule contain?
two covalent bonds
dot and cross diagram and stick diagram for water molecule
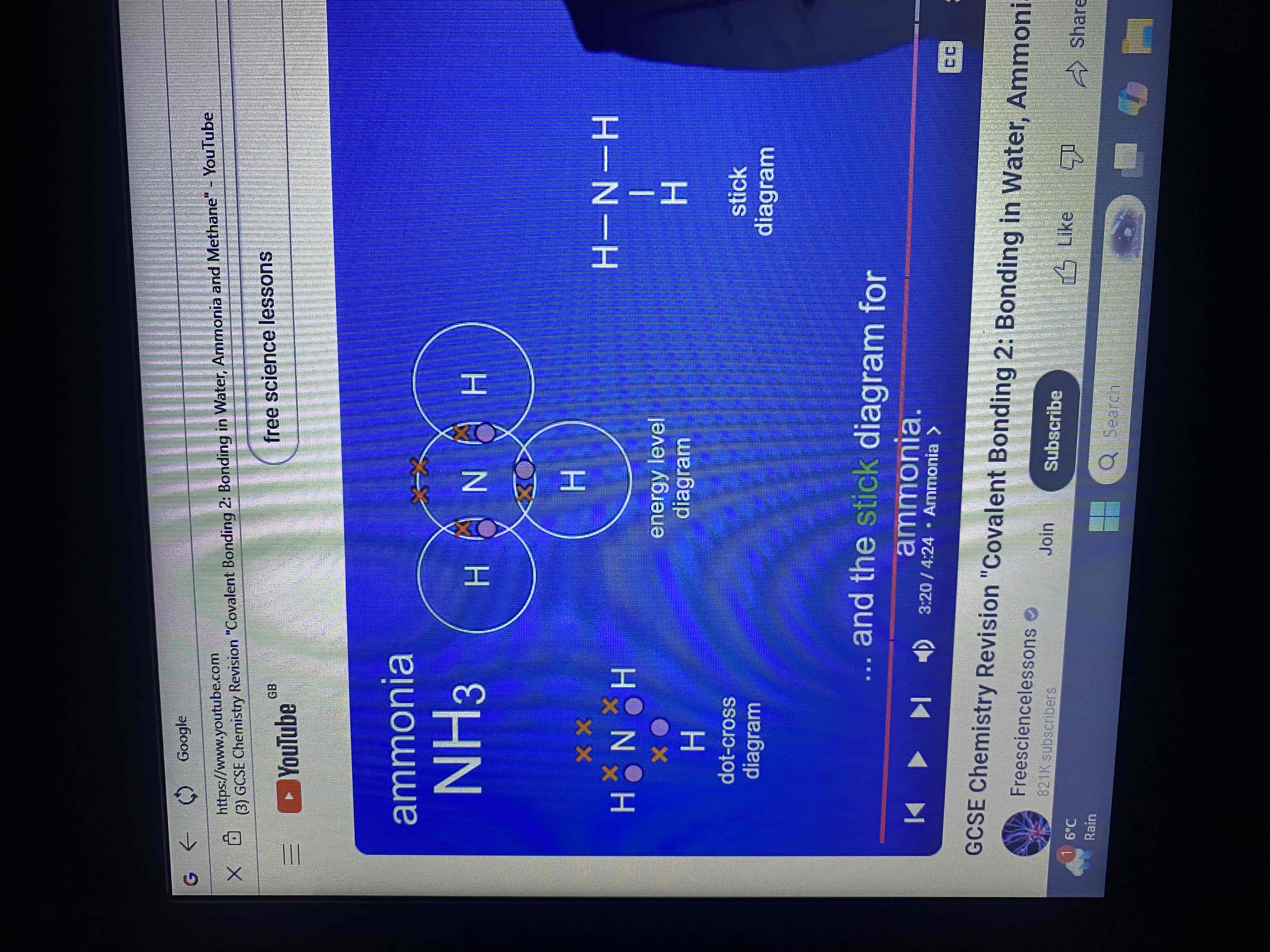
ammonia has a formula NH3 what does this tell us?
this tells us that an ammonia molecule contains one atom of nitrogen and three atoms of hydrogen.
how many electrons does nitrogen have in its outer energy level?
five electrons
energy level diagram for ammonia
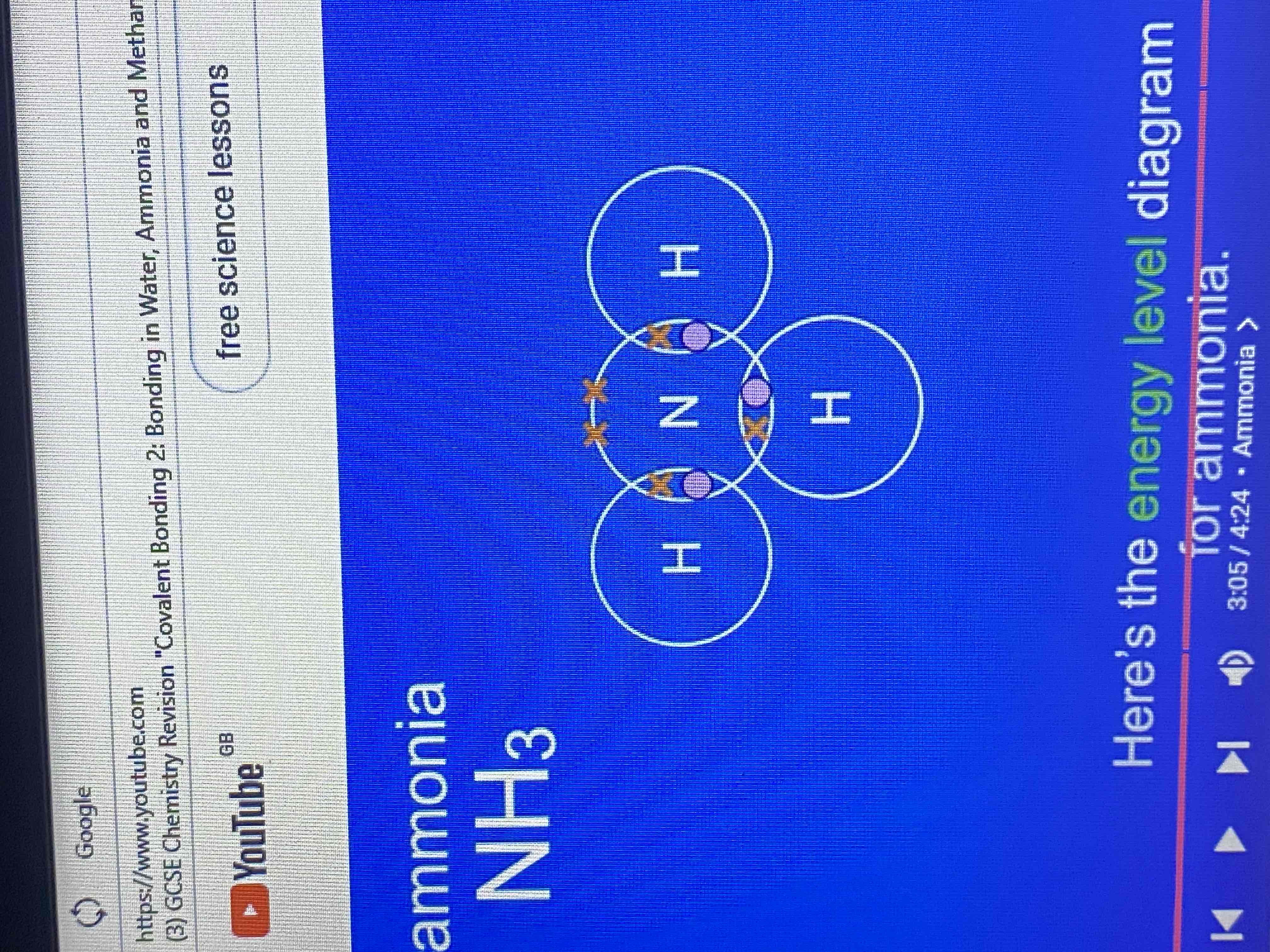
how many covalent bonds does a molecule of ammonia have?
three covalent bonds
by sharing electrons the nitrogen atom and the hydrogen atoms have each achieved what?
a full outer energy level
dot and cross and stick diagram for ammonia
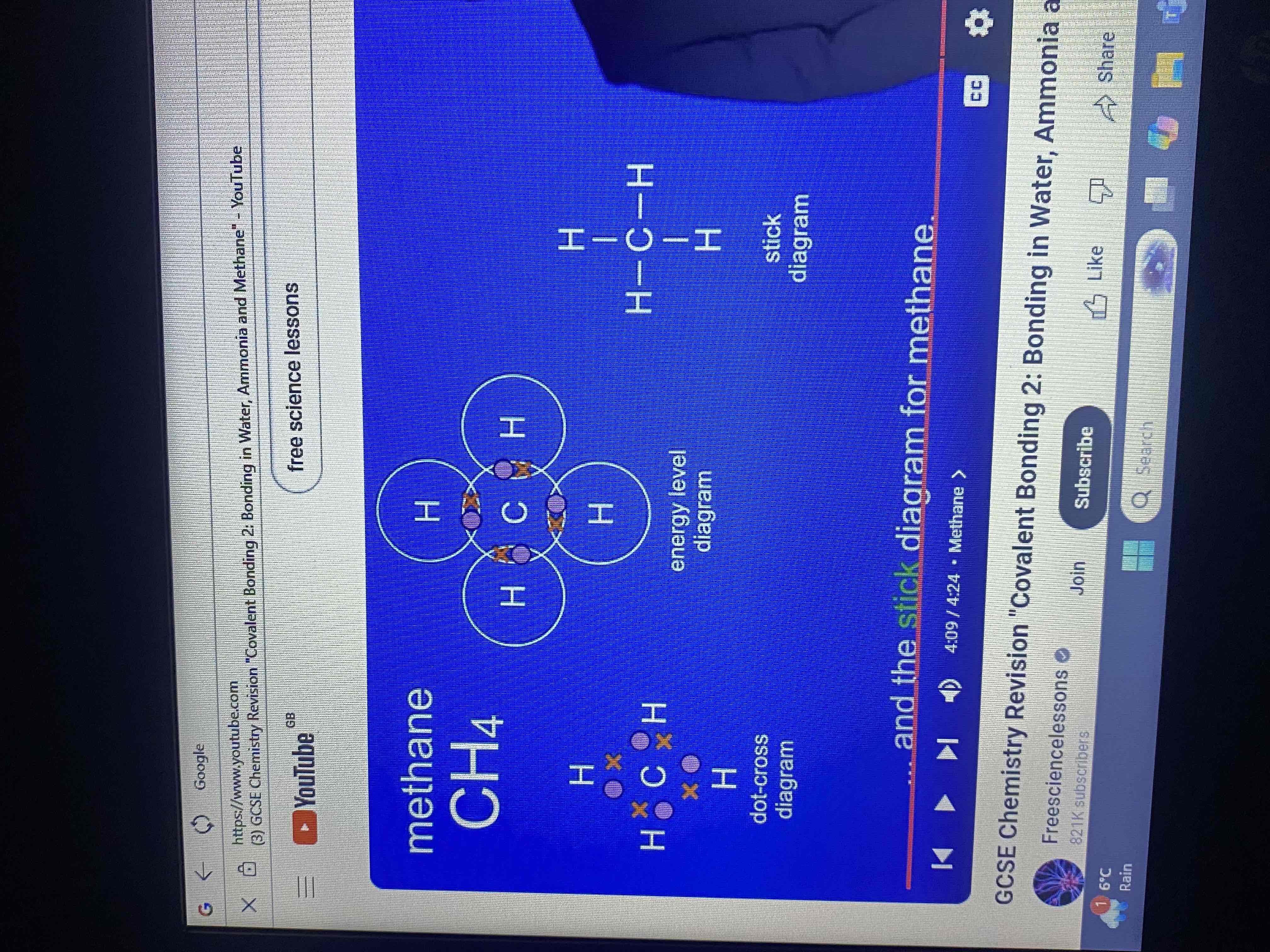
methane has a formula CH4 what does this tell us?
that a methane molecule contains one atom of carbon and four atoms of hydrogen.
how many electrons does carbon have in its outer energy level?
four electrons
energy level diagram for methane (covalent bonding in methane molecule)
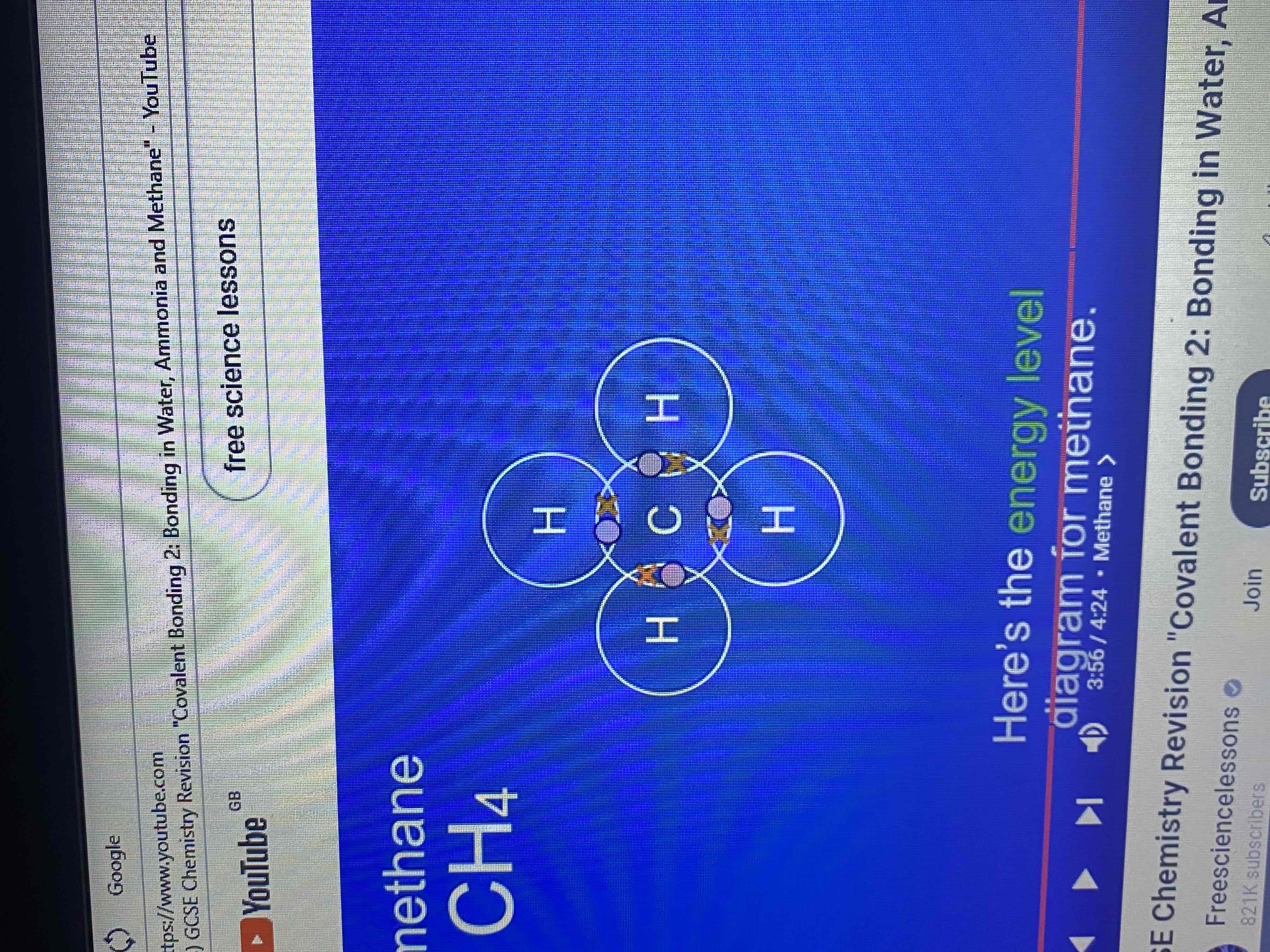
how many covalent bonds does a molecule of methane have?
four covalent bonds
what do the hydrogen atoms and carbon atom now have?
full outer energy levels
dot and cross diagram and stick diagram for methane