10.1-10.4 Acids, Bases and Salts
Properties of Acids
- Acids have pH values of below 7, have a sour taste (when edible) and are corrosive
- In acidic conditions blue litmus paper turns red and methyl orange indicator turns red
- Acids are substances that can neutralise a base, forming a salt and water
- When acids react, they will lose electrons to form positively charged hydrogen ions (H+)
- The presence of H+ ions is what makes a solution acidic
Example: Hydrochloric Acid
- HCl (aq) → H+ (aq) + Cl- (aq)
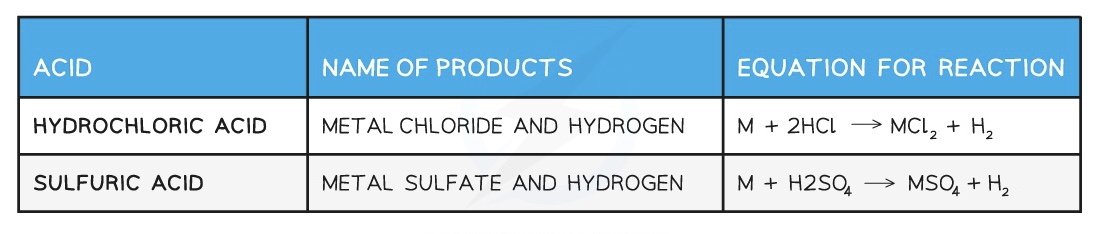
Typical reactions of acids
Acids and metals
- Only metals above hydrogen in the reactivity series will react with dilute acids.
- When acids react with metals they form a salt and hydrogen gas:
- Acid + Metal → Salt + Hydrogen
- The name of the salt is related to the name of the acid used, as it depends on the anion within the acid.
Examples of the names of salts from specific acids and metals are:
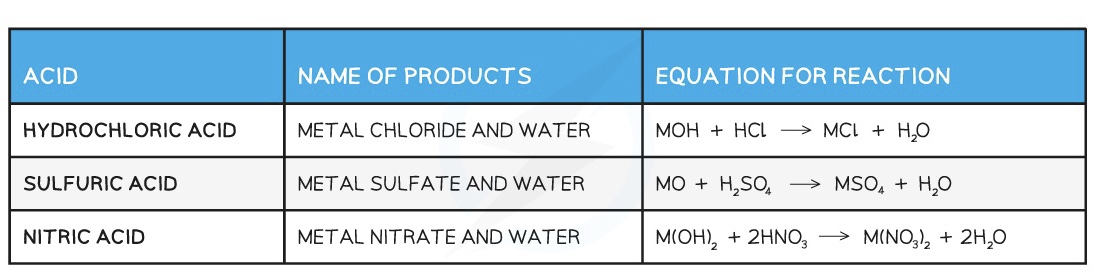
Metal oxides and metal hydroxides (alkalis) can act as bases
When they react with acid, a neutralisation reaction occurs
In all acid-base neutralisation reactions, salt and water are produced
Acid + Base → Salt + Water
Examples of reactions between acids and bases:

Acid with metal carbonate
Acids will react with metal carbonates to form the corresponding metal salt, carbon dioxide and water:
Acid + Metal Carbonate → Salt + Carbon Dioxide + Water
Examples of reactions between acids and carbonates:

Properties of Bases
- Bases have pH values of above 7
- A base which is water-soluble is referred to as an alkali
- In basic (alkaline) conditions red litmus paper turns blueand methyl orange indicator turns yellow
- Bases are substances which can neutralize an acid, forming a salt and water
- Bases are usually oxides or hydroxides of metals
- When alkalis react, they gain electrons to form negative hydroxide ions (OH-)
- The presence of the OH- ions is what makes the aqueous solution an alkali
- Example: Sodium Hydroxide
- NaOH (s) → Na+ (aq) + OH- (aq)
Typical reactions of bases
Bases and acids
When bases react with an acid, a neutralisation reaction occurs
Acids and bases react together in a neutralisation reaction and produce a salt and water:
Acid + Base → Salt + Water
Examples of reaction between bases and acids:
Alkalis and Ammonium salts
- Ammonium salts undergo decomposition when warmed with an alkali
- Even though ammonia is itself a weak base, it is very volatile and can easily be displaced from the salt by another alkali
- A salt, water and ammonia are produced
Example:
NH4Cl + NaOH →NaCl + H2O + NH3
- This reaction is used as a chemical test to confirm the presence of the ammonium ion (NH4+)
- Alkali is added to the substance with gentle warming followed by the test for ammonia gas using damp red litmus paper
- The damp litmus paper will turn from red to blue if ammonia is present
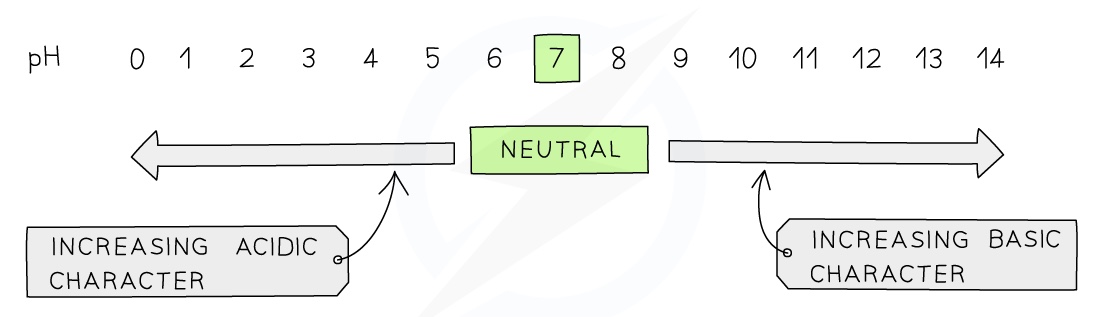
The pH scale
The pH scale is a numerical scale which is used to show how acidic or alkaline a solution is
It goes from 1 - 14 (extremely acidic substances can have values of below 1)
All acids have pH values of below 7, all alkalis have pH values of above 7
The lower the pH then the more acidic the solution is
The higher the pH then the more alkaline the solution is
A solution with a pH of 7, such as water, is described as being neutral
Universal Indicator
Universal indicator is a mixture of different indicatorswhich is used to measure the pH
A drop is added to the solution and the colour is matched with a colour chart which indicates the pH which matches specific colours

The importance of pH and Soil Acidity
- Soil pH is analysed to indicate the acidity or alkalinity of soil
- Most plants favour a pH value of between 5 and 8
- Changes in soil which cause a pH to be outside this range adversely affect plant processes resulting in reduced growth and crop yield
- Soils may become acidic from acid rain, overuse of fertilisers which contain ammonium salts or by the excessive breakdown of organic matter by bacteria
- Crushed or powdered limestone (calcium carbonate) or lime (calcium oxide) or slaked lime (calcium hydroxide) is added to neutralise the excess acidity in the soil
- The addition process must be carefully monitored though, as if added in excess, further damage could be done if the pH goes too high
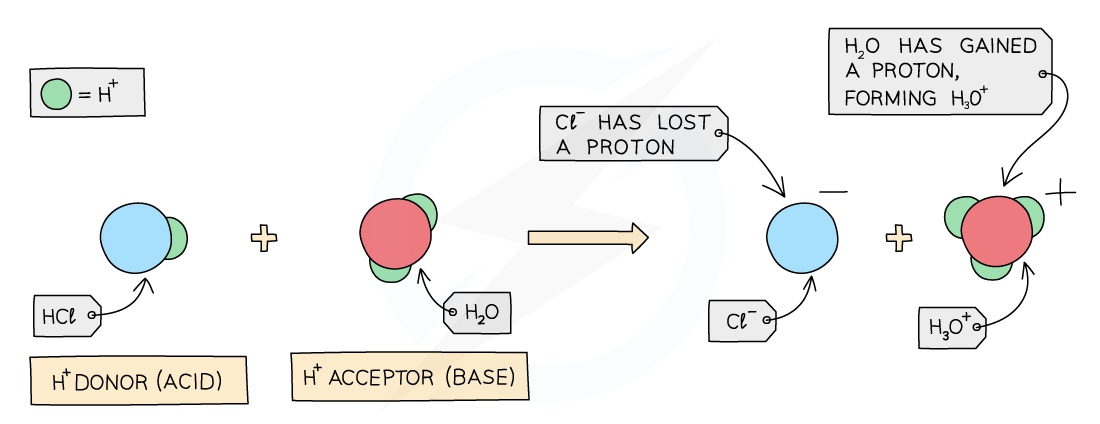
Proton Transfer, Weak & Strong Acids & Bases
Proton Transfer
- The earlier definition of an acid and a base can be extended
- In terms of proton transfer, we can further define each substance in how they interact with protons
Acids
- Acids are proton donors as they ionise in solution producing protons, which are H+ ions
- These H+ ions make the aqueous solution acidic
Bases (Alkalis)
Bases (alkalis) are proton acceptors as they ionise in solution producing OH- ions which can accept protons
These OH- ions make the aqueous solution alkaline
^^Strong Acids and Bases^^
- Acids and alkalis can be either strong or weak, depending on how many ions they produce when dissolved in water
- Strong acids and bases ionise completely in water, producing solutions of very low pH for an acid or very highpH for a base
- Strong acids include HCl and H2SO4 and strong bases include the Group I metal hydroxides
%%Weak acids and bases%%
- Weak acids and bases partially ionise in water and produce pH values which are closer to the middle of the pH scale
- Weak acids include organic acids such as ethanoic acid, CH3COOH and weak bases include aqueous ammonia
- For both weak acids and bases, there is usually an equilibrium set-up between the molecules and their ions once they have been added to water
- Example of a weak acid: propanoic acid
CH3CH2COOH ⇌ H+ + CH3CH2COO-
- Example for a weak base: aqueous ammonia
NH3 + H2O ⇌ NH4+ + OH-
- In both cases the equilibrium lies to the left, indicating a high concentration of intact acid / base molecules, with a low concentration of ions in solution
Effect of concentration on strong and weak acids and alkalis
- A concentrated solution of either an acid or a base is one that contains a higher number of acid or base moleculesper dm3 of solution
- It does not necessarily mean that the acid or base is strong though, as it may be made from a weak acid or base which does not dissociate completely
- For example a dilute solution of HCl will be more acidic than a concentrated solution of ethanoic acid, since most of the HCl molecules dissociate but very few of the CH3COOH do
The Oxides
what are oxides?
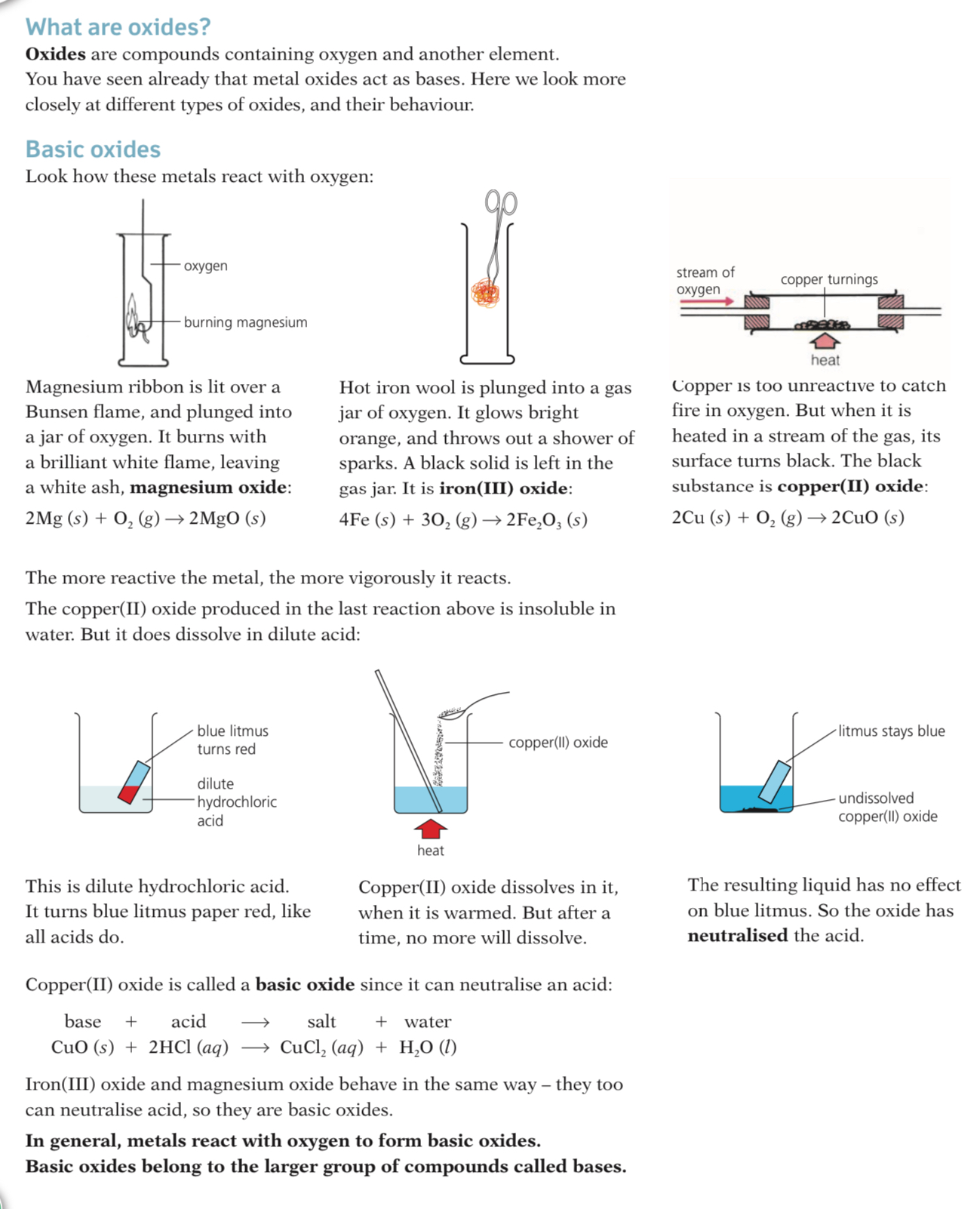
Oxides are compounds containing Oxides and another. element you have seen already that metal oxides act as bases, here we look more closely at different types of oxides and their behavior.
Acid and basic Oxides
Acidic and basic oxides have different properties and values of pH
The difference in their pH stems from whether they are bonded to a metal or a non-metal element
The metallic character of the element influences the acidic or alkaline behaviour of the molecule
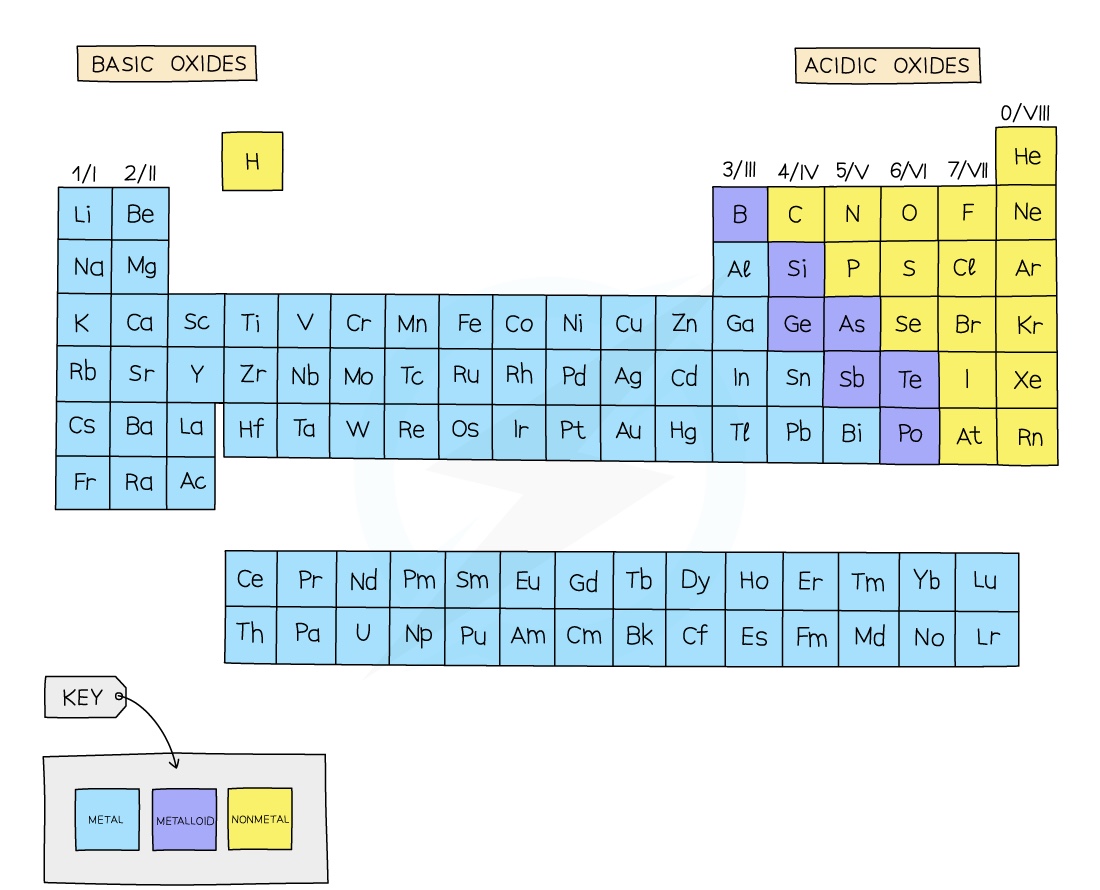
Acidic Oxides
Acidic oxides are formed when a non-metal element combines with oxygen
They react with bases to form a salt and water
When dissolved in water they produce an acidic solution with a low pH
Common examples include CO2, SO2, NO2 and SiO2

Basic oxides
Basic oxides are formed when a metal element combines with oxygen
They react with acids to form a salt and water
When dissolved in water they produce a basic solution with a high pH
Common examples include NaOH, KOH and Ca(OH)2
IGCSE- EXTENDED- Neutral Oxides
- Some oxides do not react with either acids or bases and thus are said to be neutral
- Examples include N2O, NO and CO
Amphoteric oxides
- Amphoteric oxides are a curious group of oxides that can behave as both acidic and basic, depending on whether the other reactant is an acid or a base
- In both cases a salt and water is formed
- Two of the most common amphoteric oxides are zinc oxideand aluminum oxide
- The hydroxides of both of these elements also behave amphoterically
- Example of aluminium oxide behaving as a base:
Al2O3 + 6HCl → 2AlCl3 + 3H2O
- Example for an aluminium oxide behaving as an acid:
Al2O3 + 2NaOH → 2NaAlO2 + H2O
- This acidic and basic behaviour is not easily explained by the donating or accepting protons. A separate theory called the Lewis acid-base theory can identify acids or bases in these situations, but is not required for this course
Preparation Of Salts
Salts
- A salt is a compound that is formed when the hydrogen atom in an acid is replaced by a metal
- For example if we replace the H in HCl with a potassium atom, then the salt potassium chloride is formed, KCl
- Salts are an important branch of chemistry due to the varied and important uses of this class of compounds
- These uses include fertilisers, batteries, cleaning products, healthcare products and fungicides
Naming salts
- The name of a salt has two parts
- The first part comes from the metal, metal oxide or metalcarbonate used in the reaction
- The second part comes from the acid
- The name of the salt can be determined by looking at the reactants
- For example hydrochloric acid always produces salts that end in chloride and contain the chloride ion, Cl-
- Other examples:
- Sodium hydroxide reacts with hydrochloric acid to produce sodium chloride
- Zinc oxide reacts with sulfuric acid to produce zincsulfate
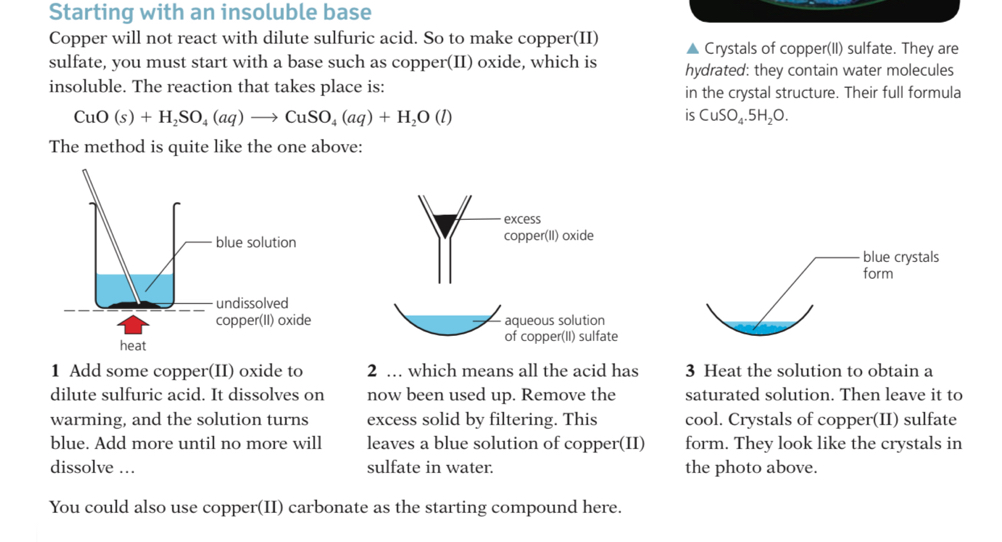
- There are two key ideas to consider when preparing salts:
- Is the salt being formed soluble or insoluble in water?
- Is there water of crystallisation present in the salt crystals?
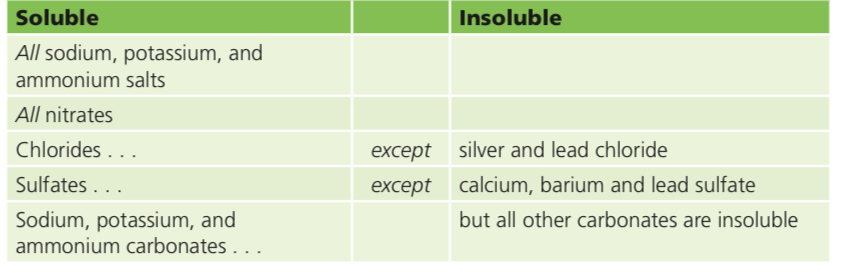
Solubility of the common salts


Not all salts are soluble:
Insoluble salts= The salts we looked at so far have all been soluble. You could obtain them as crystals, by evaporating solutions. But not all salts are soluble. This table shows the ‘rules’ for the solubility of salts:


Identification of Ions
Identification of Cations
- Metal cations in aqueous solution can be identified by the colour of the precipitate they form on addition of sodium hydroxide and ammonia
- If only a small amount of NaOH is used then normally the resulting metal hydroxide precipitates out of solution
- In excess NaOH some of the precipitates may re-dissolve
- A few drops of NaOH is added at first and any colour changes or precipitates formed are noted
- Then the NaOH is added in excess and the reaction is observed again
- The steps are then repeated for the test using ammonia solution
Analysing results
- The table below contains the results for each of the cations included in the syllabus
- If a precipitate is formed from either NaOH or aqueous ammonia then it means that the hydroxide is insoluble in water
- Zinc chloride, for example, reacts as such:
ZnCl2 (aq) + 2NaOH (aq) → Zn(OH)2 (s) + 2NaCl (aq)
Ca2+ ions can be distinguished from Zn2+ and Al3+ as calcium hydroxide precipitate does not dissolve in excess NaOH but both zinc hydroxide and aluminium hydroxide do
Zn2+ ions can be distinguished from Al3+ ions as Zn(OH)2dissolves in excess aqueous ammonia but Al(OH)3 does not
Most transition metals produce hydroxides with distinctive colours
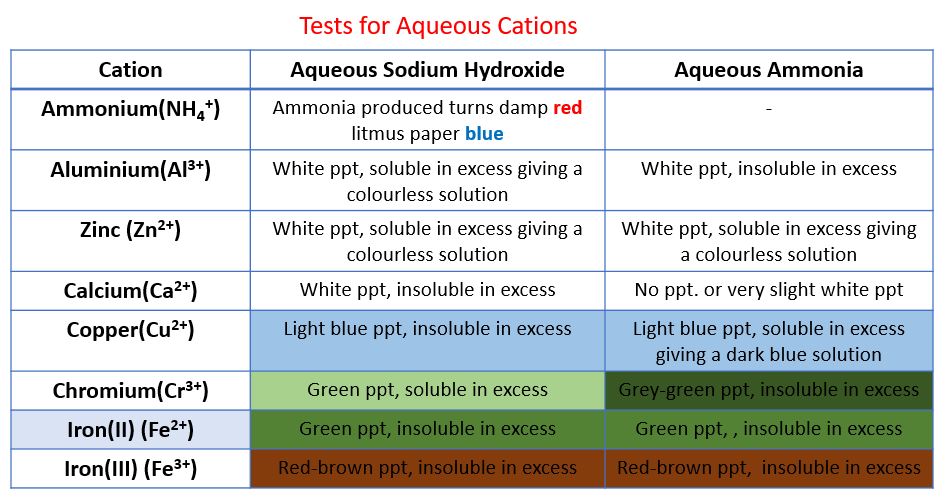
Test for Cations
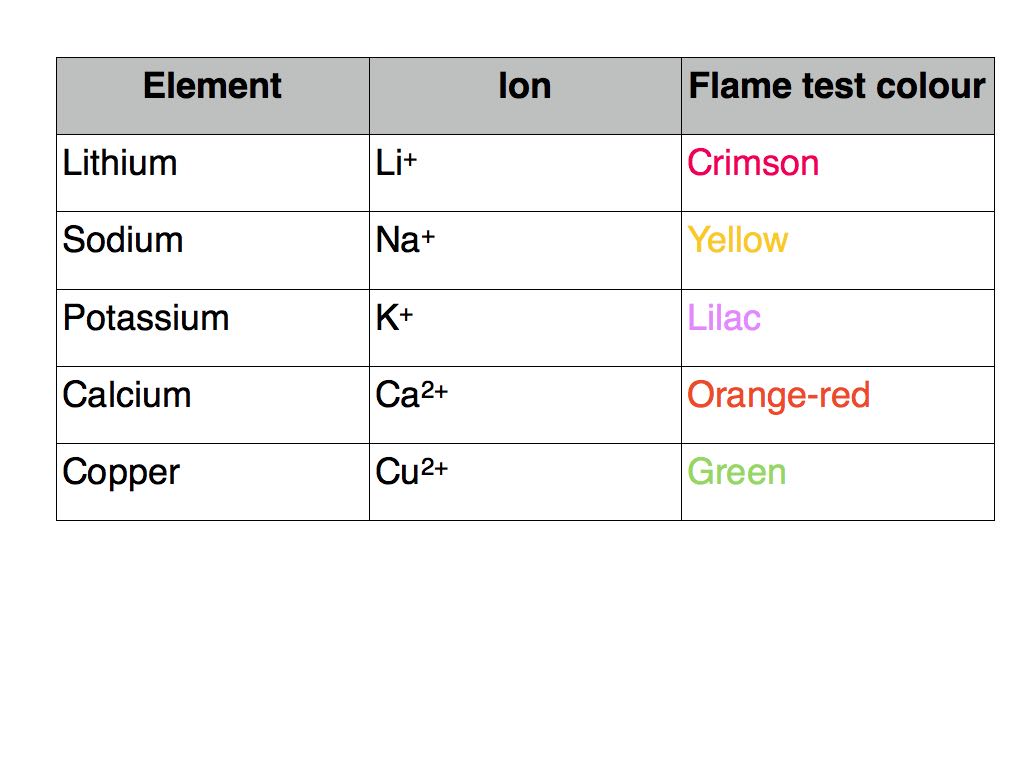
- The flame test is used to identify the metal cations by the colour of the flame they produce
- A small sample of the compound is placed on an unreactive metal wire such as nichrome or platinum
- The colour of the flame is observed and used to identify the metal
Identification of Anions
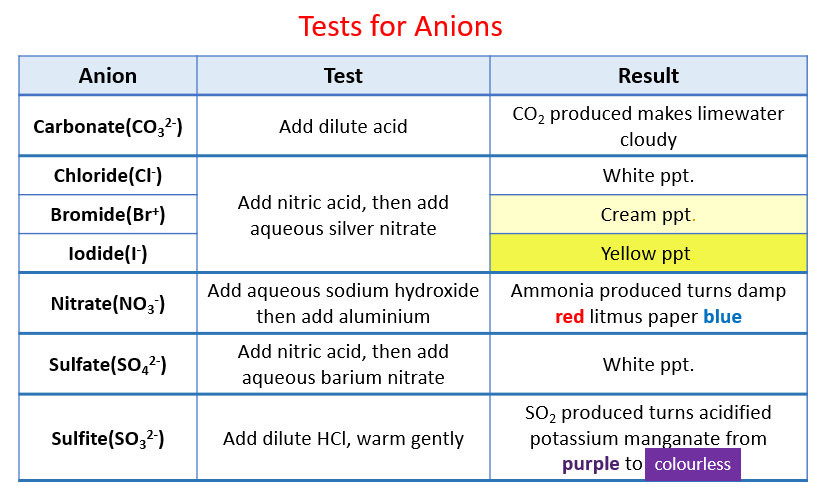
Identification of Gases
Several tests for anions and cations produce gases which then need to be tested
The table below indicates the tests for the gases included in the syllabus

\n \n