Neur201 module 4 (Special senses)
1/97
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
98 Terms
Somatosensory system
Provides info to the brain about the state of the body and external environment. This info is used to guide behaviour and maintain homeostatic function
Special senses
Vision, hearing, balance, smell, taste
Lobes of the brain, brocas & Wernicke’s area, cortexes
Temporal= auditory,
Frontal= motor, prefrontal cortex
Primary motor, then somatosensory and below that is primary taste area
Occipital= vision, visual cortex

Sensory map
is distorted because sensitive regions (high receptor density and small receptive fields) occupy a large area of somatosensory cortex
Somatosensory cortex, 2-point discrimination
Large area (large sensory input)= lips, tongue, hands
Small area (2 sharp objects would feel like 1)= elbow, neck
2 sharp object poked into skin, closer they can be when they can be discriminated= more receptive fields
Motor efferent information flow
CentralNS to PeripheralNS
Somatic= muscles, voluntary
Autonomic= sympathetic (Noradrenaline) or parasympathetic (acetylcholine)
Sensory affarent info flow
PeripheralNS to CentralNS
Info from receptors to spinal nerves to brain & spinal cord
Dorsal vs ventral peripheral nerve
Dorsal root (affarent info)
sensory info comes in
Cell body is in dorsal root ganglion (collection of cell bodies)
Ventral horn (efferent info)
Cell bodies of motor neurons are in the spine
Axon goes to muscle
Interneuron in the spinal cord links the dorsal to the ventral

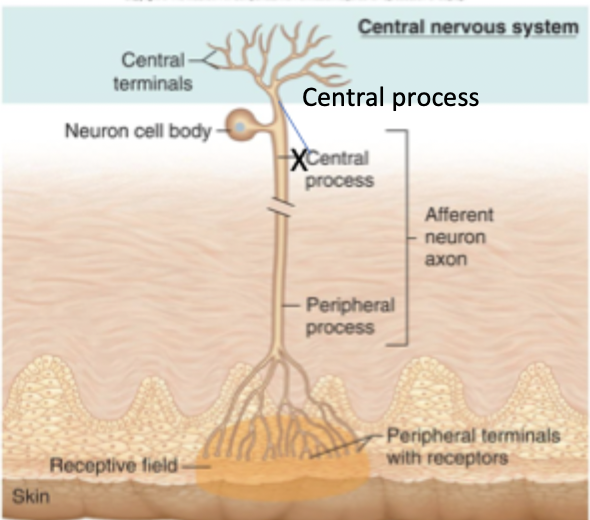
Sensory neurons (primary affarent neurons)
Cell body is typically located in the dorsal root ganglia or cranial nerve ganglia
Peripheral process ends at receptive location. Nerve endings in the periphery are dendrites.
Central process enters the CNS
area that dendrites form branches defines receptive field
Nerve endings can be free or encapsulated
Synapses form on secondary sensory neurons
Somatosensory receptors/neurons can encode 4 main features of the stimulus
Modality= receptor specificity (e.g. touch and temp are reported by diff receptors)
Intensity= frequency of AP firing in a sensory axon and the number of activated axons determines strength of stimulus
Location= Somatosensory mapping of receptors in specific areas allows for location of the stimulus to be known
Duration= beginning/end pattern of AP firing can encode the start and end of a stimulus
Receptive field
The area where stimulation affects activity of the neuron. Size relates to two-point-discrimination.
Receptor adaptation
receptors can be rapid or slow adapting
Stimulus intensity pathway
Stimulus intensity is controlled by the frequency of action potentials, not their amplitude.
Stronger stimuli generate larger receptor potentials (changes in membrane potential), which cause more frequent action potentials that propagate and release more neurotransmitter into the CNS.If depolarisation reaches threshold voltage gated Na+ channels are activated
More time spent above threshold= more AP generated
Explain this image
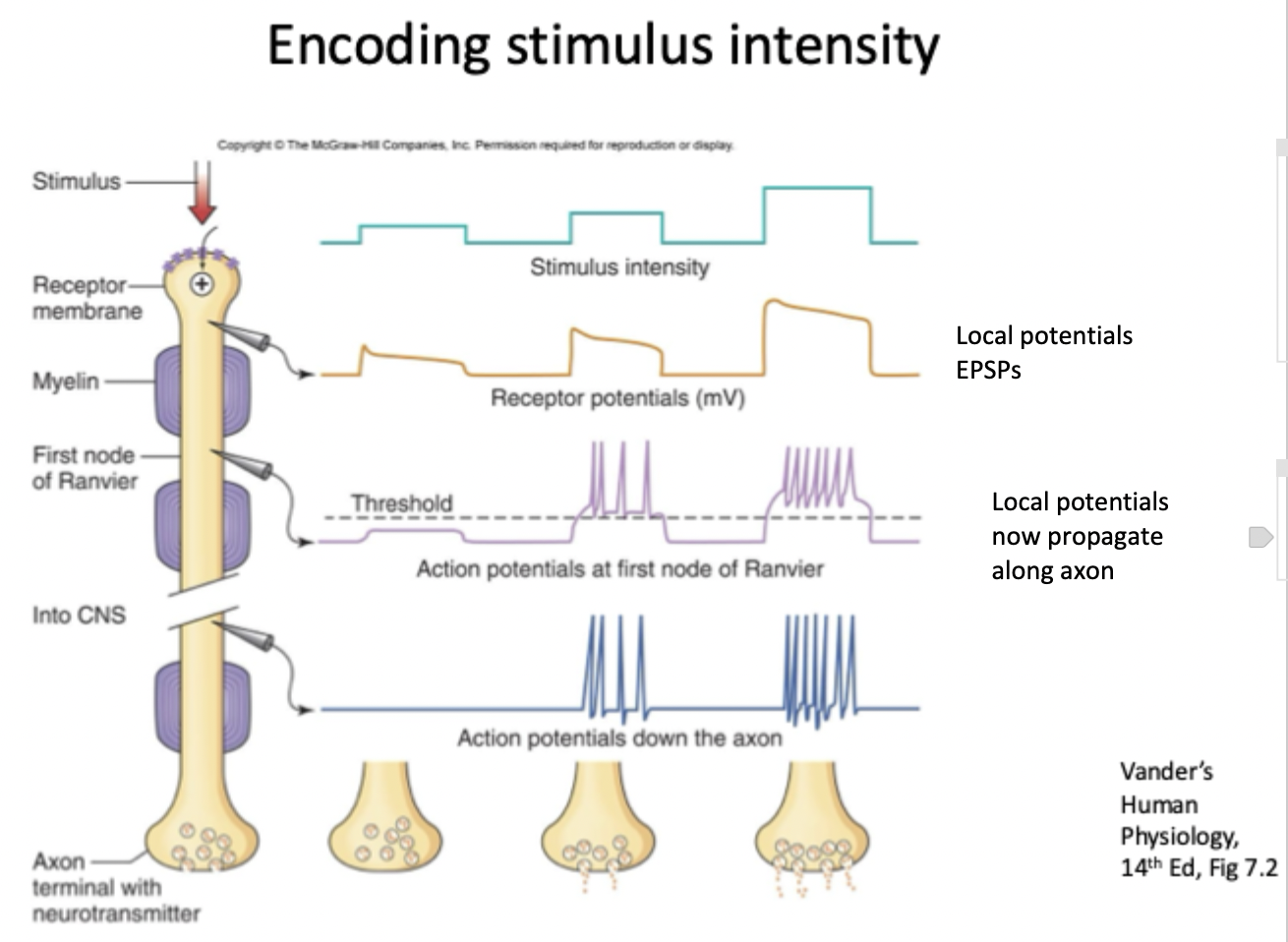
Stimulus intensity: as the intensity of the stimulus increases the neuron translates it into a signal that the CNS can interpret.
Receptor potentials: Graded (local potentials) at the receptor membrane, not an AP yet, stronger the stimulus= larger receptor potential, local depolarisations (EPSPs) that can summate.
AP at first node of ranvier: When receptor potential (changes in MP) reaches threshold AP fires. Increased stimulus intensity= increased frequency of AP (size is the same every time).
AP down the axon: AP travels down the axon through saltatory conduction (jumping from node to node bc of myelin)
Axon terminal w neurotransmitter release: AP triggers influx of Ca2+ and neurotransmitter release. More frequent AP= more neurotransmitter release= stronger postsynaptic response.
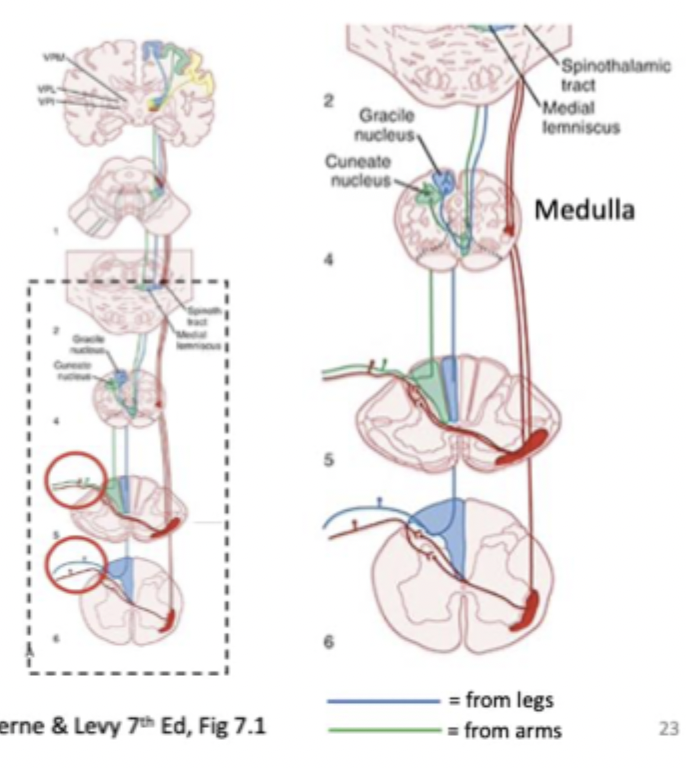
Ascending somatosensory pathway 1 (Dorsal column)
= fine touch and vibration
Input through dorsal root
synaptic contact (crossing over) in the medulla after ascending up the spine
Because of the crossing, sensations are experienced on the opposite side of the cortex
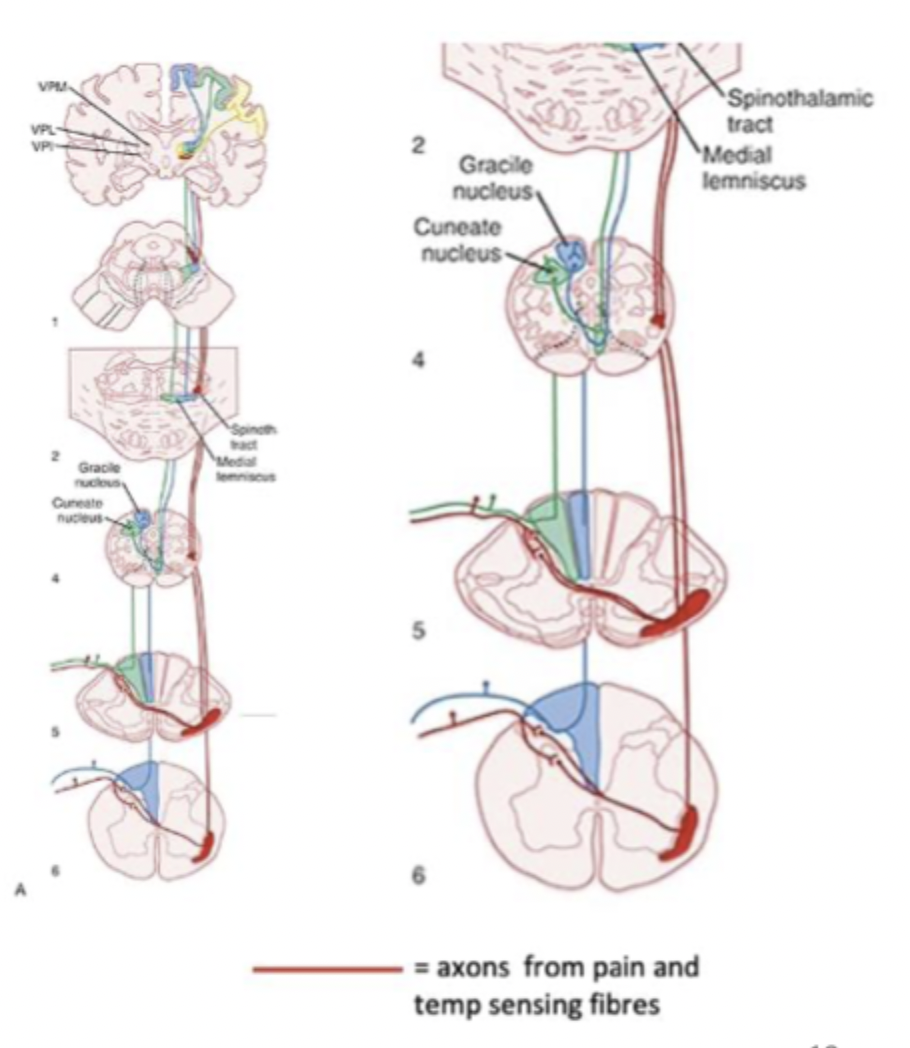
Ascending somatosensory pathway 2 (spinothalamic tract)
= pain, temperature, non-discriminative touch
Pain and temperature axons synapse in the dorsal spinal cord
Spinal cord neurons go across the midline and then ascend to thalamus
Thalamus projects to somatosensory cortex
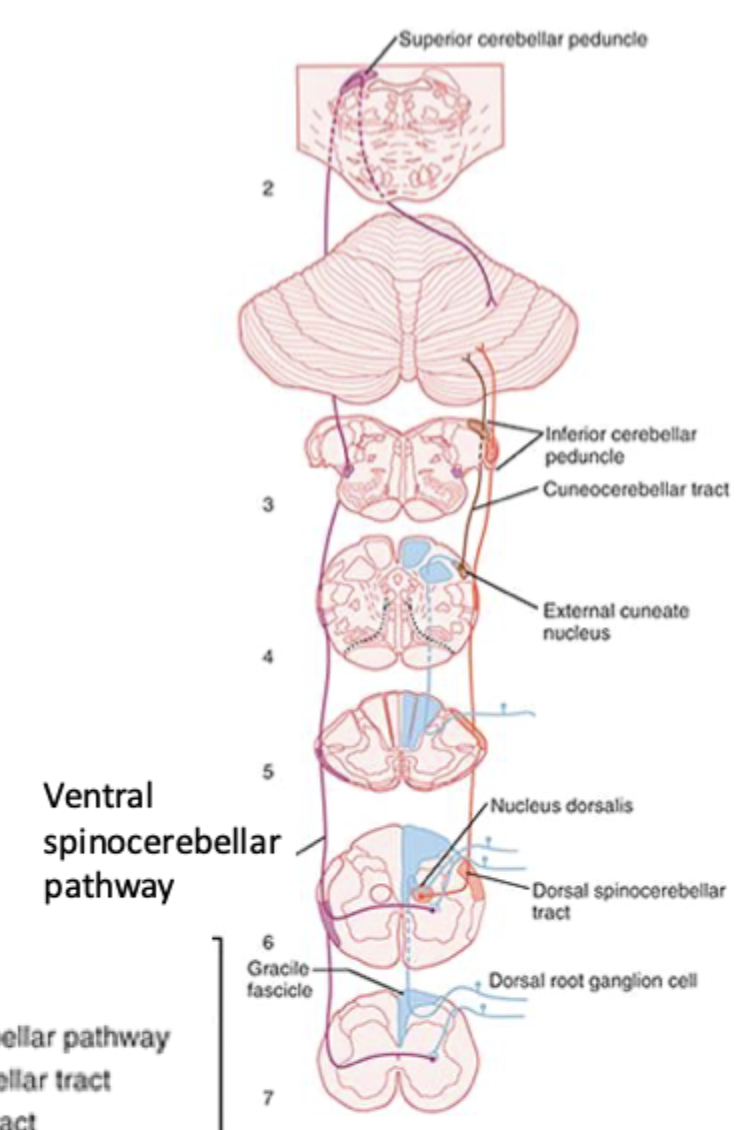
Ascending somatosensory pathway 3 (Spinocerebellar tracts)
= proprioception
Balance and movement are initiated in the cerebellum
Position sensing receptors go to the cortex and cerebellum via spinocerebellar tract
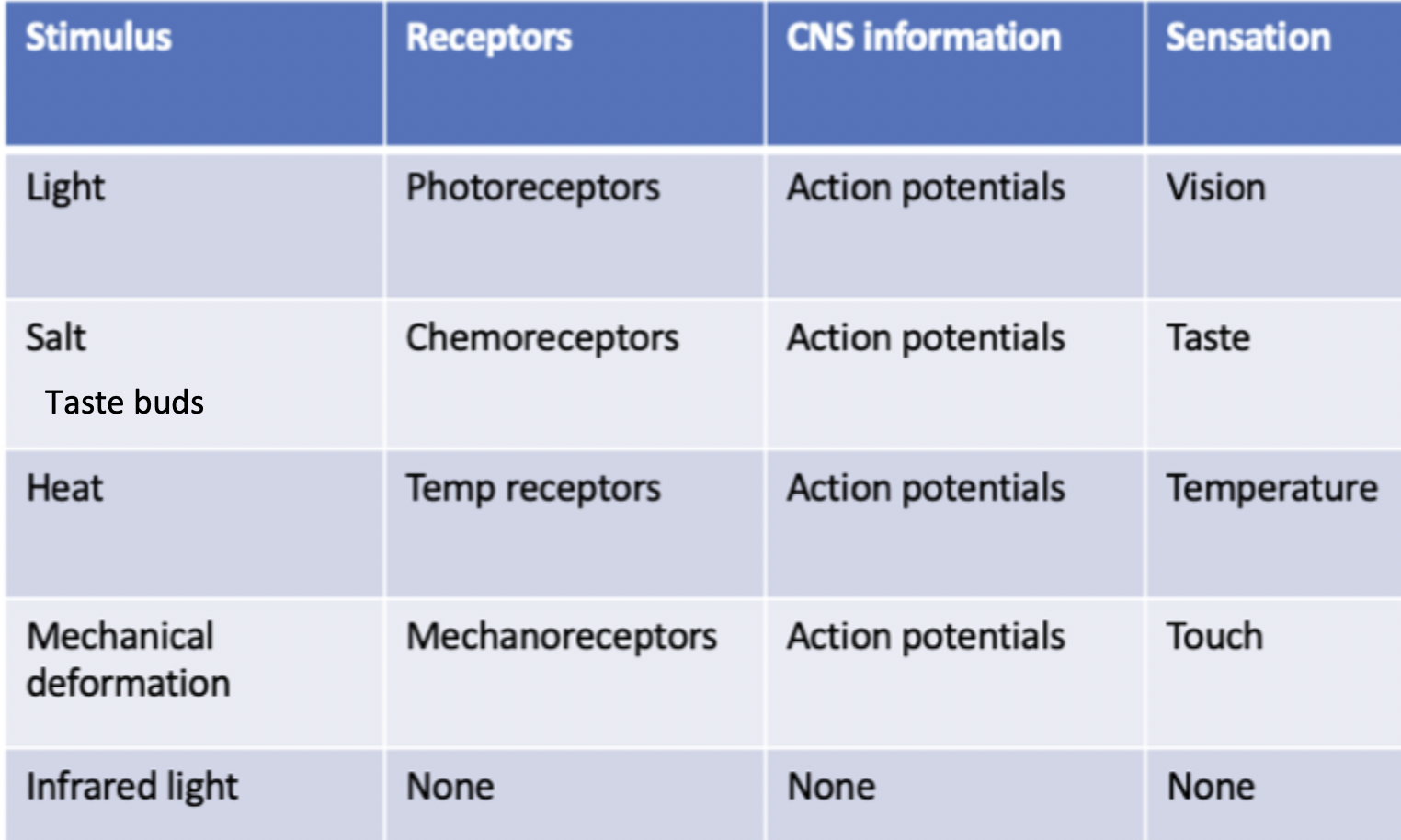
Sensory signal transduction receptors
Photoreceptors= Light stimulus, vision
Chemoreceptors= salt stimulus, taste
Temp receptors= heat stimulus, temperature
Mechanoreceptors= mechanical deformation stimulus, touch
None= infared light stimulus
Types of chemoreceptors
Chemoreceptors are sensory cells with receptors that respond to presence of a specific chemical
Taste & smell= E.g. salty taste NaCl, sour taste Acid
Peripheral chemoreceptors= Aortic & carotid bodies detect pCO2, [H+] and O2 in the blood. Crutial in control of breathing
Central chemoreceptors= On the surface of medulla, detect pH of cerebralspinal fluid.
Visual spectrum
400-750nm
Optical & neural components of the eye
Optical= front of the eye (e.g. lens, cornea), collects and focuses light into the plane of the retina.
Neural= Converts energy of light into changes in membrane potential. Other parts of the brain decode it to generate visual perceptions.
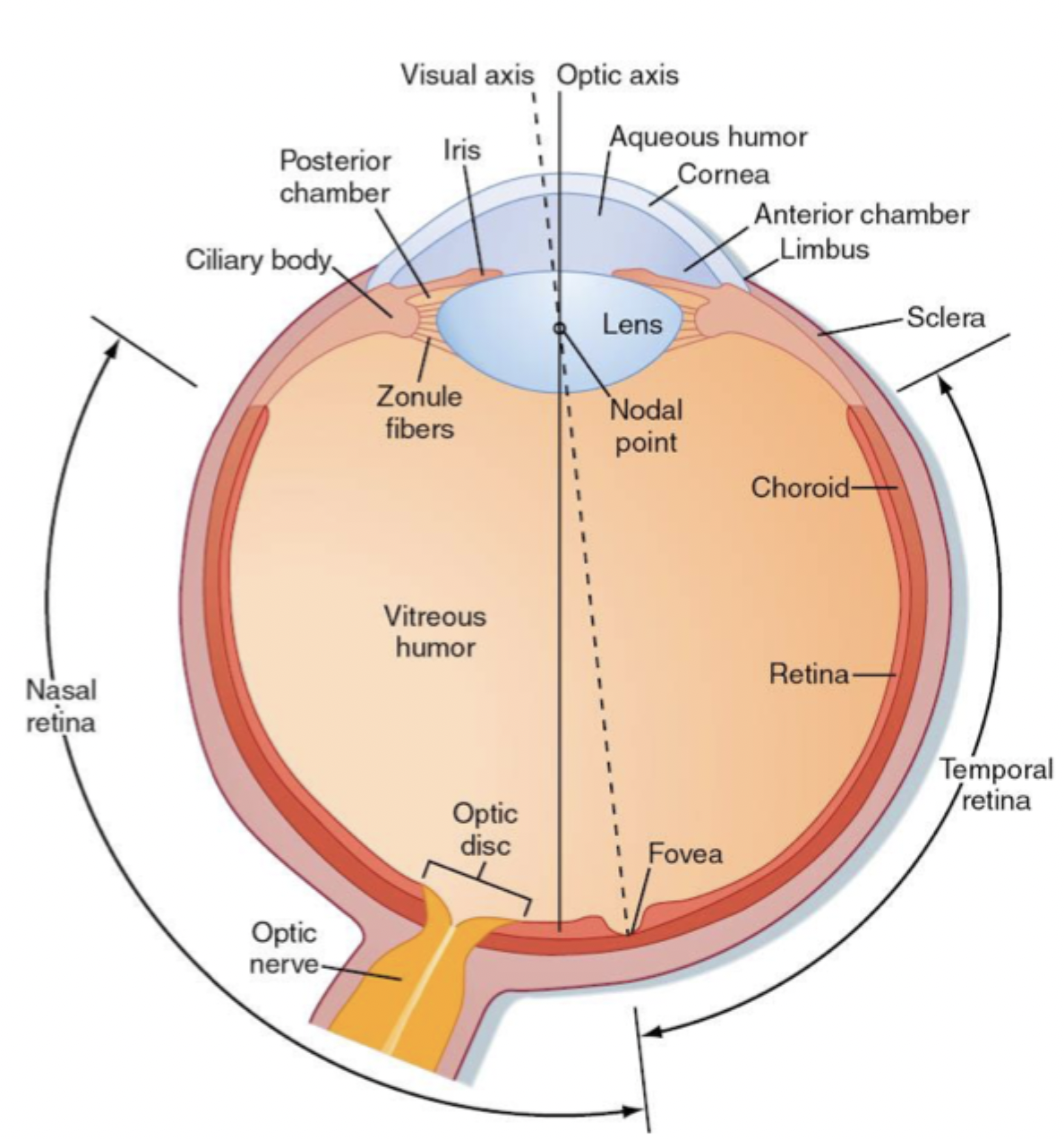
Parts of the eye (Cornea, Aqueous humour, Lens, Zonule fibres, Ciliary body)
Outer/outward facing layer of the eye
Jelly-like layer that maintains pressure to keep the eye round
Separates aqueous humour from the vitreous humour, allows us to have near and far focus, controls how much light enters the eye
Thin and strong fibres that connect lens to ciliary body
Round muscle (sphincter), pulls zonule fibres when relaxed, shrinks when it contracts. Contraction allows the eye to focus on a near object, uses the centre of the eye for the most detail.
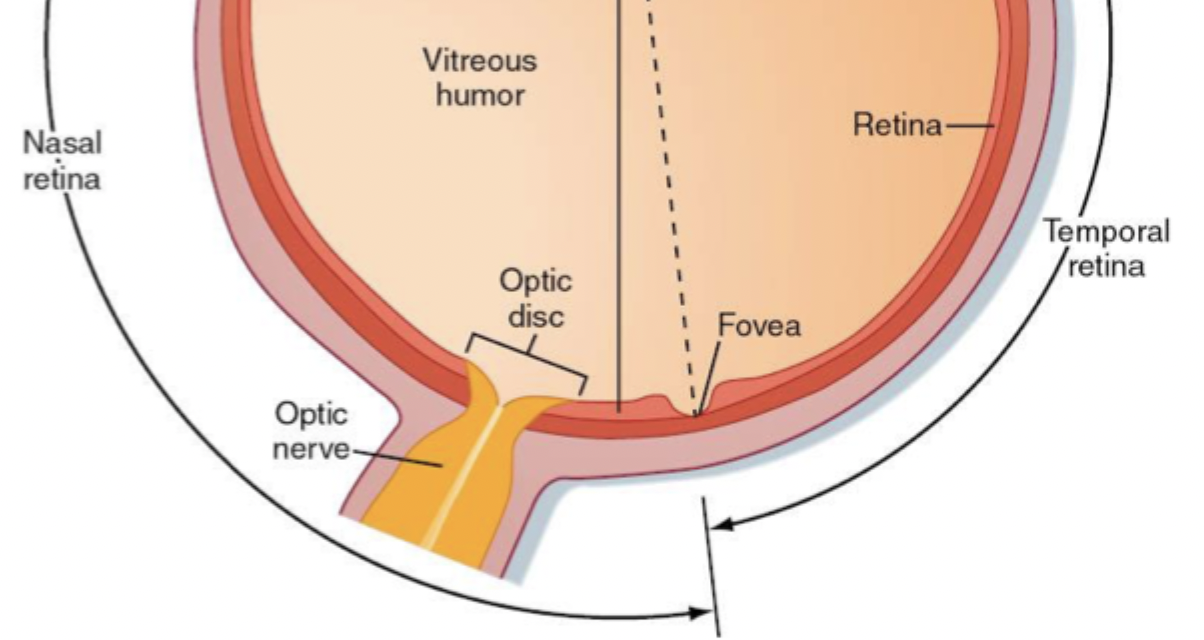
Retina, optic disk, fovea
Where rods, cones and photoreceptors are. Where photons are turned into AP before being sent to primary visual cortex.
Part of nasal retina where neurons pass their axons into the brain and where blood vessels come out. Doesn’t have photoreceptors, where blind spot is.
The most detail is gained when the light is focused on the fovea
Refraction in the eye
How image gets into the eye. Light travels between mediums of refractive indexes.
Results in change in direction of light, related to difference in refractive indices of 2 media and curvature of refractive surface.
Retina is a curved surface so all the images are upside down and back to front. The brain switches this around
3 Processes when looking from distance to near objects
Accomodation= contraction/relaxation of ciliary muscle to alter lens shape and refractive power
Constriction of pupil= excludes edges of the lens to improve focus
Convergence of eyes= eyes moved closer together so objects remain in register on corresponding parts of the two retinae (especially foveae)
Ciliary muscle relaxing and contracting
= Accomodation
Relaxing= when looking at far away objects decreased parasympathetic activity of ciliary muscle, zonular fibres are taut (stretched, more tension), lens is flattened
Contracting= when looking at near objects increased parasympathetic activity of ciliary muscle, decreased tension in zonular fibres, lens more spherical, refractive power and angle of refraction increases
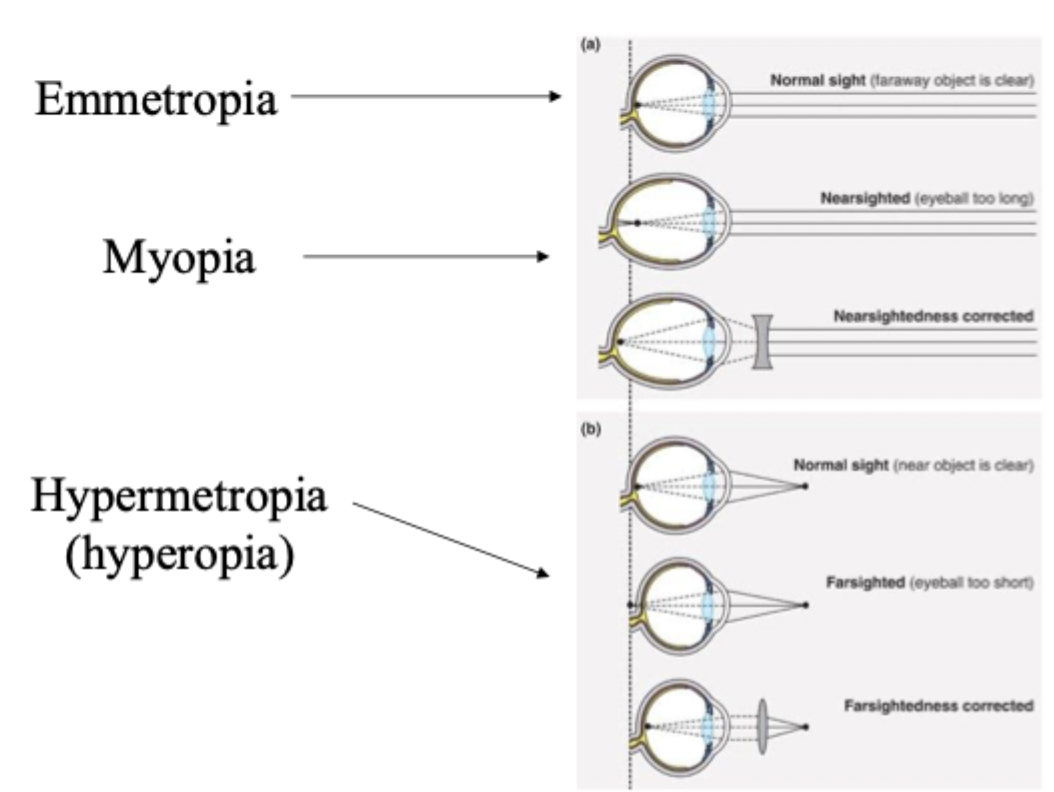
Emmetropia, myopia, hypermetropia/hyperopia
normal vision
Eyeball too long, near sighted. Far object focus is infront of retina. Concave lens pushes focus (decrease convergence)backward onto retina. Near sight is fine.
Eyeball too short, far sighted. Far sight: is fine because of accomodation but convex lens causes muscles to relax. Near sight: has insufficient accomodation so convex lens brings the focus forward (increase convergence).
Presbyopia, Astigmatism, Caracat
Age related loss of accomodation, lens loses elasticity, far sight is good, near sight is impaired so need convex lens to correct vision
Curvature of lens and/or cornea is is not sphere which causes multiple focal points (leads to blurry vision) , different amount of refraction in diff planes, corrected with cylindrical lens
Lens opaque, lens broken in capsule so plastic intraocular lens installed, lens surgery causes inability to accommodate glasses are needed.
Pathway that light passes through in the retina
Ganglion layer —> bipolar & horizontal cells —> photoreceptors (rods & cones, change in membrane potential and glutamate)
Photorecepters, Rods vs cones, photopigment
Disks increase surface area, inner segment helps cell to survive
120mil per retina, few in fovea, low light (night vision), disks free floating, photopigment rhodopsin
8mil per retina, lots in fovea (focus), high light, colour vision, disks joined by membrane, photopigment photopsin (blue/S, green/M, red/L)
Opsin + retinal from vitamin A
Phototransduction in the dark vs light
Retinal not activated (inactive cis isoform), lots of cGMP, cGMP channels open, Na+ & Ca2+ influx, Photoreceptor depolarised (-35mV), lots of glutamate released onto bipolar cells
Light energy, retinal changed to active trans isoform, G protein activates cGMP phosphodiesterase which breaks down cGMP, less cGMP & cGMP gated channels close, Less Na+ & Ca2+ influx, photoreceptor hyper polarised (-60mV), less glutamate released onto bipolar cells
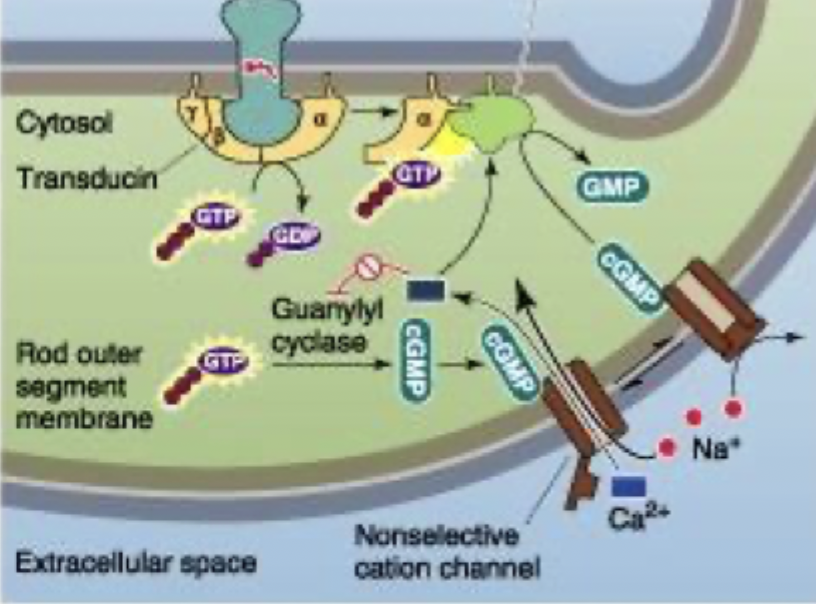
Phototransduction process
Guanylyl cyclase turns ATP→ cGMP which is cis isoform of retinal so active in the dark
cGMP activates non-selective cation channel that allows Na+ and Ca2+ to come into cell
Photoreceptor depolarised (-35mV) and releases glutamate onto bipolar cells
Colour wavelength
Blue cone (small), then Rod, then Green (medium) cone, then red (large) cone.
Cone doesn’t cross with Red spectra
Colour blindness
Can be congenital (inherited, 8% of males, 0.5% of females) or aquired (disease)
Genes for production of M & L opsin (red/green sensitivity/inability to distinguish between) are on the X chromosome.
This means since females have an extra one it can correct the colour blindness in the other
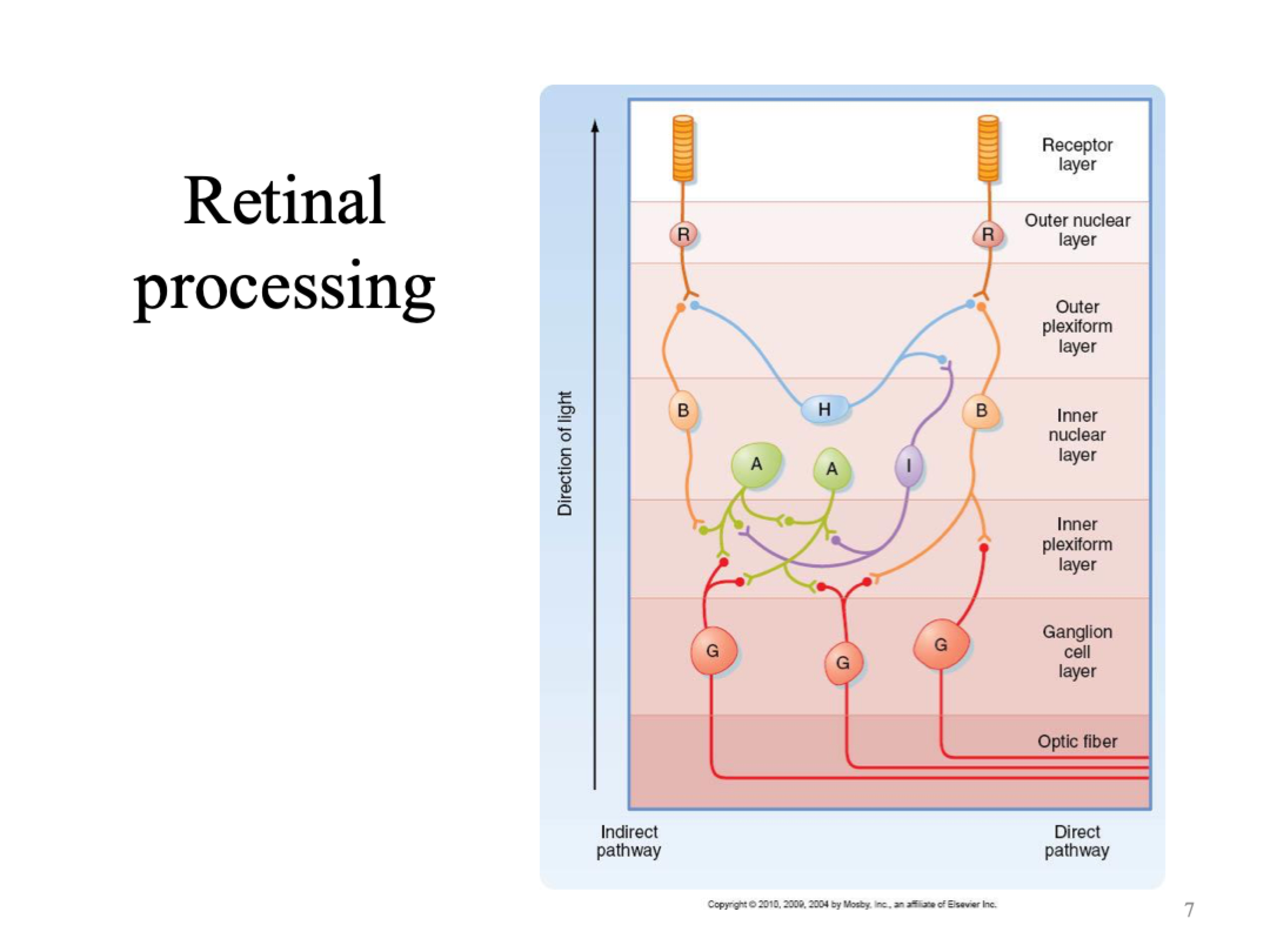
Light interacts with photoreceptors which causes slight hyperpolarisation (change in local graded potential) which influences the cells below and modulates membrane potential of those cells around them.
Ganglion cells are the only one of these cells that fire action potentials
Ganglion cell receptive fields (on centre vs off centre)
On centre field responds to central light, light in periphery reduces AP firing rate, increased AP firing when light is in centre
Off centre field responds to light in the periphery, reduced response to central light, increased AP firing when there is light in periphery
The overlap and sum of ganglion cell receptive fields determines where edges are and the image that is seen.
Visual acuity
Low convergence ganglion cell receives info from a small number of cones. Small receptive fields (at fovea/centre of retina)= high acuity= pick up detail well
High convergence ganglion cell receives info from a large number of photoreceptors (rods & cones). Large receptive fields= doesn't know which photoreceptor photon is from= less detailed vision
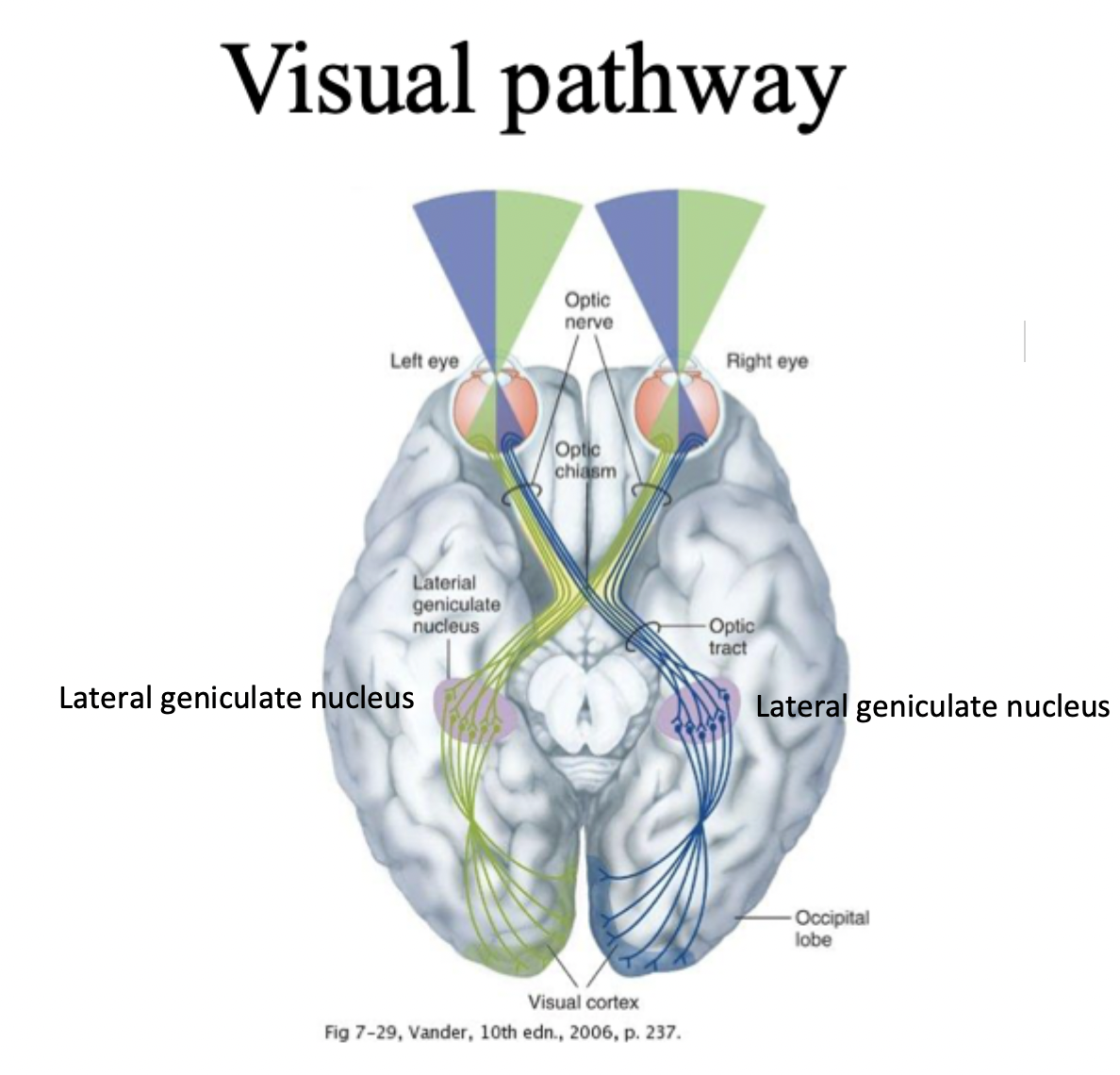
Nasal retina, temporal reina, lateral geniculate nucleus
Nasal retina= side closest to nose, info goes to opposite side of brain (contralateral) by crossing at optic chasm
Temporal retina= side closest to ear, info goes to same side of brain (ipsilateral)
Lateral geniculate nucleus= where processing occurs before being sent to occipital lobe
Ganglion cell axons project to four main subcortical visual areas
Superior Colliculus= eye movements and orientation to visual stimuli
Lateral geniculate nucleus= sensation of vision
Pretectum= control of pupils
Superchiasmic nucleus= control of dinural rhythms
Loudness
Number of action potentials fired, longer you are exposed to high decibels the more likely you are to lose hearing
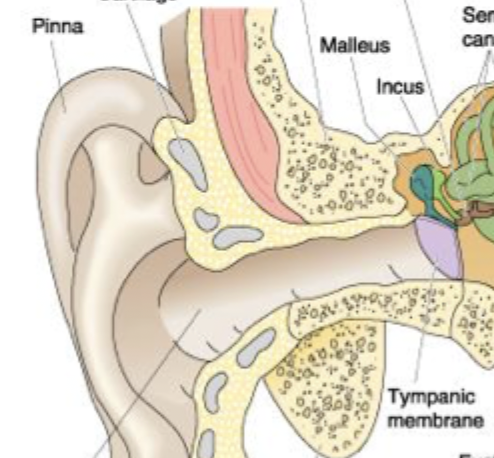
Pinna,ear canal, tymphatic membrane
Pinna captures air waves, Sound waves go through the ear canal and cause tymphatic membrane to flex
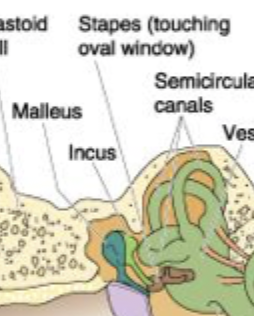
Malleus, incus, stapes
= small bones that generate pressure on the oval window because energy goes from Malleus→Incus→stapes
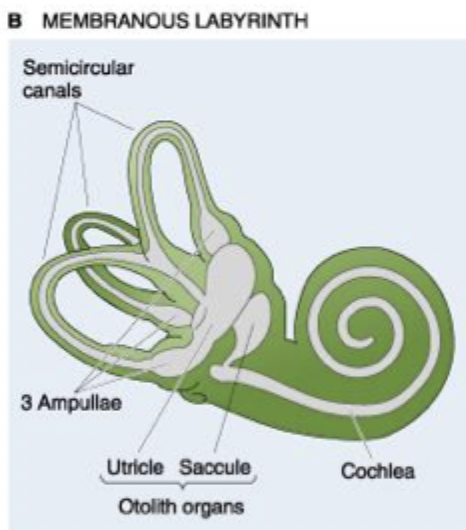
Membranous labyrinth
Cochlea= Interprets what sound waves are telling us, looks like snail
Oolith organs (utricle & saccule), tell us if we are accelerating up/down/forwards/backwards.
3 ampullae
Semicircular canals= independent of each other, don’t share the same fluid, tells us if our head tilts
Energy transfers from ear to fluid (pathway through ear),
Tymphatic membrane deflects
Ossicles/Middle ear bones (malleus, incus, stapes) move, damage to middle ear can affect balance
membrane in oval window moves
Basilar membrane moves
Tuning of basilar membrane
= specialised hair cells, flexible
High pitch sound= basilar membrane oscillates close to oval window (thin, narrow and stiff end)
Low pitch sound= basilar membrane vibrates closer to apex (wide and floppy end with high frequency)
Deflection of stereocilia
Vibration of basilar membrane at a specific point causes deflection of stereo cilia on the hair cells that are above.
Hair cells become depolarised at a location along the basilar membrane that corresponds to the pitch of the sound
Organ of corti, Basilar membrane, tectorial membrane
How we hear things
has specialised hair cells, flexible
runs along entire length of cochlea apparatus and sits above hair cells, doesn’t vibrate but hair cells move left or right
Signal transduction in hair cells, positive vs negative mechanical deformation
hair cells are caused to move by the vibration of basilar membrane
Pitch of the sound causes hair cells to be activated along a specific part of the BM which triggers firing of affarent axon at a location that corresponds to the pitch of the sound
Kinocilium, Stereocilia, tiplink, positive vs negative mechanical deformation
Biggest hair cell
Other hair cells
Tip that links together kinocilium and sterocilia
Positive= tip links bend toward kinocilium, mechanically gated K+ channels open, K+ flow into cell, depolarisation, AP firing
Negative= tip links bend away from kinocilium, mechanically gated K+ channels close, hyper polarisation
Sound qualities (Pitch, intensity, duration, direction)
frequency, discrimination determined by activity in hair cells at specific points on basilar membrane
number of fibres activated & AP per second in auditory nerve fibres
duration of affarent discharge caused by stimulus
time difference indicated by activation of receptors & intensity differences in each ear (sound arrives in one ear and then the other to determine direction of sound)
Tonotopic organisation
Info from sensory axons originating on hair cells close to the oval window projects to neurons in posterior region of auditory cortex so part of the cortex is responsive to high pitch sounds and vice versa
both sides of the brain get info from both ears and map it together
Deafness, conduction deafness, sensorineural deafness
= raised threshold to sound stimuli, damaged hair cells
Impaired sound transmission through outer or middle ear, can be due to blockage or infection
Damage to receptor or neural pathways, can be due to exposure to sound noises, antibiotics, illness
Vestibular functions, vestibular-ocular reflex
generates reflexes to compensate for head movement and the perception of motion in space
promote stable images on the retina during head movements (vestibular nystagmus, eyes compensating for movement)
provides info to allow conscious awareness of position/movement/acceleration
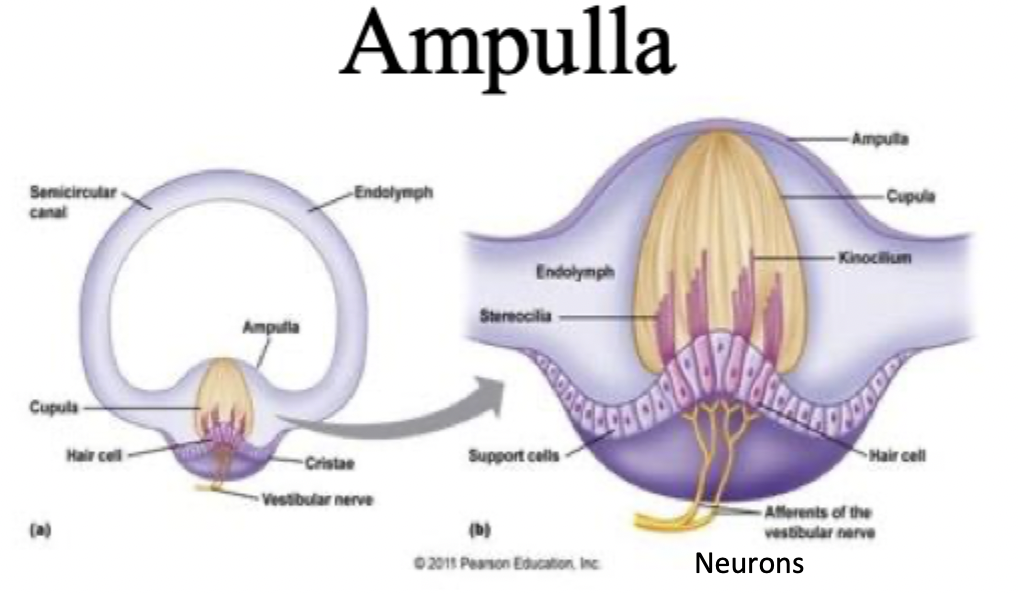
Ampulla, Cupulla, kinocillium, acceleration, spinning
Each semicircular canal has one (3 semicircular canals on each side=6)
yellow, jelly-like matrix
Purple hair cells, can depolarise/hyperpolarise
relative movement of the enclosed endolymph fluid pushes against cupula which displaces the hairs, causing opening of mechanically gated ion channels
When spinning fluid has a delay, so head movement in one direction causes fluid to push against cupula momentarily in the other direction (decrease in AP production because of inertia)
Hair cells during head movement, not bent, bent toward kinocilium (depolarisation), bent away from kinocilium
vestibular cells have a resting rate of discharge, rate of discharge can increase or decrease when head moves
Individual hair cells can signal movement in 2 directions
Baseline firing
K+ channels open, K+ flow into cell, increased firing. Depolarisation opens VG Ca2+ channels, vesicles fuse, neurotransmitter release onto affarent nerve fiber
Close K+ channels, less K+ into cell (but there are still leak channels), hyper polarisation, decreased firing
Head movement, Move head to right vs after stopped
Left and right hear work together
Stereocilia on the right more active than left, increased firing in right horizontal canal, decreased in left
Decreased firing in right, increased in left
Oolith organs
Utricle= horizontal when standing, info on horizontal linear acceleration
Saccule= vertical when standing, info on vertical linear acceleration
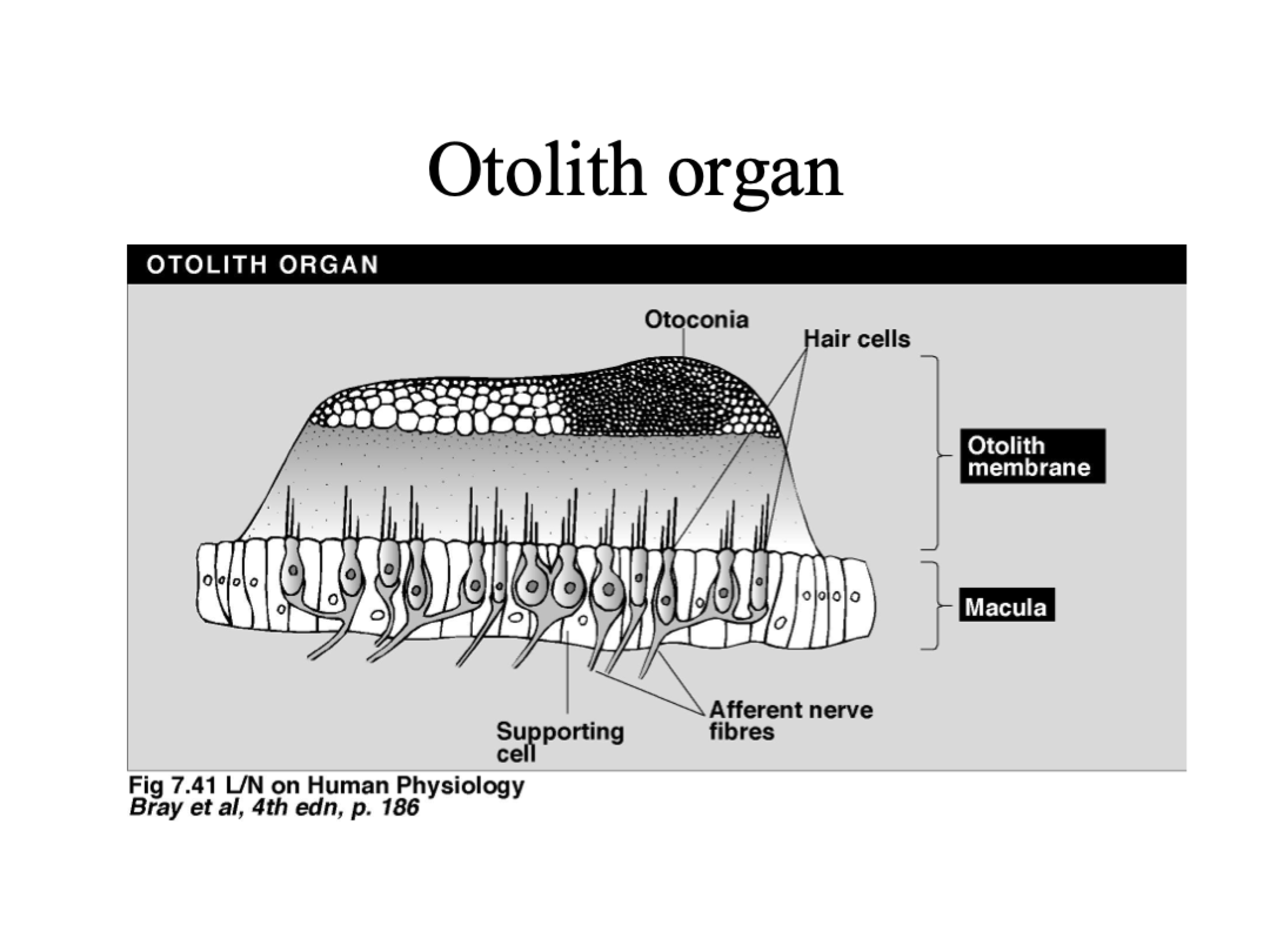
Otoconia, Accelerating to the left (left & right hairs), sudden stop, constant acceleration
Provides resistance
Left hairs= bend away from kinocilium (decrease firing rate)
Right hairs= bend towards kinocilium (increase firing rate)
Hairs and otoconia bend in opposite direction to before
Constant acceleration= no bending, baseline rate of firing
Axons from vestibular system project to vestibular nuclei in brainstem, Info from there used to:
Stabilise eyes (via oculomotor nuclei)
Stabilise head (via input to neck muscle motor neurons)
Maintain balance (via pathways to cerebellum and spinal cord)
Vertigo, motion sickness, bedspins, ototoxic drugs
Caused by diseases effecting vestibule or its affarent fibres. illusion of movement, dizziness, nausea
Mismatch between visual and vestibular info
Caused by alcohol. Ethanol infiltrates cupula, lowers density and creates perception of movement
medicines harmful to hair cells, can result in temp or permanent hearing loss and disorders of balance
Why do we have chemical senses?
sample food and drink for nutrient content, palatability, toxicity
sensory perceptions in response to ingested and inhaled chemicals
guide apetite, trigger processes for absorbing nutrients
Avoid poisons and harmful chemicals
Find a mate
Taste buds in tongue papillae
In tongue papillae, saliva traps molecules and they fall into pits
there are different types of papillae but they all have taste buds
Microvilli increase surface area
Taste buds
we have 2000-5000 taste buds (on tongue, pharynx, palate, epiglottis and small intestine)
Each taste bud is cluster of 50-100 columnar epithelial cells
Taste buds have undifferentiated “immature” taste cells
3 main cell types= Type I (yellow), II (green)and III (blue)
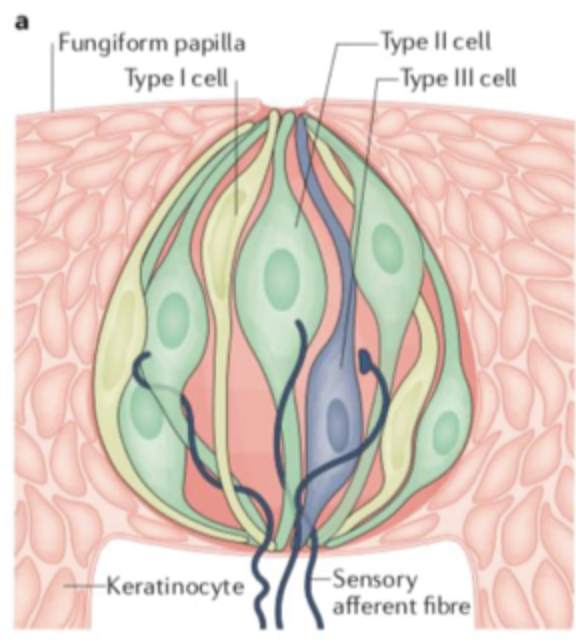
Type I, type II and type III cells (in taste buds)
Glial-like, uptake of K+ and transmitters, supportive, not directly involved in taste sensation
have chemoreceptors (GPCRs) for sugars, amino acids or bitter stimuli
neuron-like, have ion channels to sense sour and salty stimuli
Classes of taste
Sweet= stimulated by sugars
Sour= stimulated by acids
Salty= stimulated by sodium (NaCl)
Bitter= complex, typically stimulated by alkaloids, e.g. coffee, beer, wine
Umami= stimulated by amino acids (especially glutamate & aspartate), MSG= monosodium glutamate
general mechanism of taste
typically depolarising signal
tastant (ligand) reacts with receptor, which causes increase in Ca2+
Release of neurotransmitter or signalling molecule which interacts with affarent nerve fibre (AP)
Type II receptor cells have a non-vesicular mechanism of releasing signalling molecule
Sweet
Sensed by type II cells, stimulated by sugars and sugar-like molecules
receptor is a homodimeric GPCR (T1R)
receptor activation activates cephalic phase insulin release (insulin released before blood sugar rises)
Activating T1R activates G-protein complex (Gusducin) which activates PLC
PLC increases Ca2+ release from ER, depolarisation (via TRPM5) and release of signalling molecule via non-vesicular mechanism
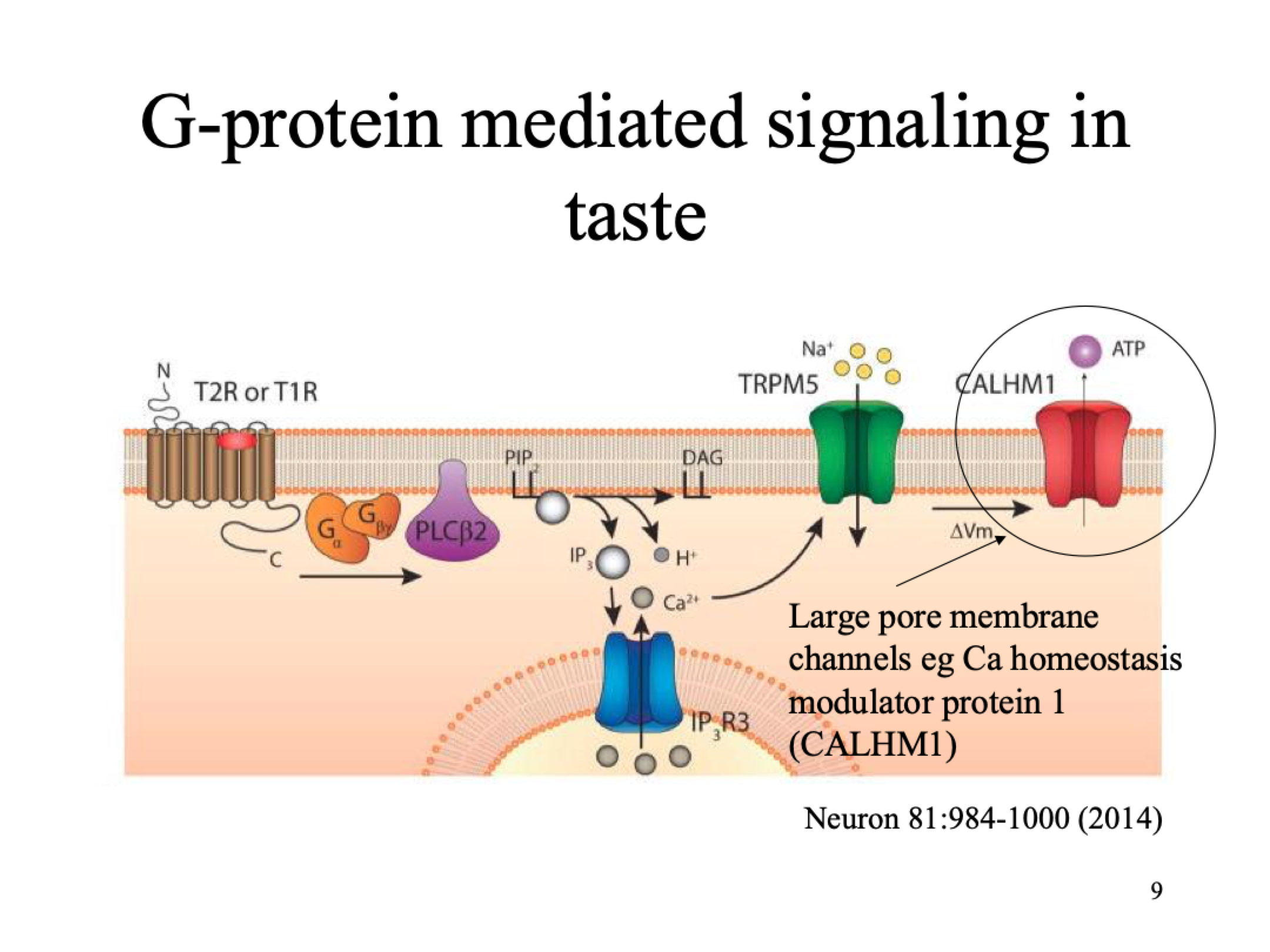
G-protein mediated signalling in taste for bitter, umami, sweet but diff receptor for each
taste receptor GPCRs:Sweet= homodimeric T1R, Umami= Heterodimeric T1R, Bitter=Monodimeric T2R
Sugar molecule binds to receptor (Gq GPCR)
PLC breaks down PIP2 into IP3 and DAG (secondary messengers that amplify signal)
IP3 goes to ER and activates R3 channel, Ca2+ conc in cell increases
TRMP5 channel activated, temp sensitive, Na+ goes into cell
Change in MP activates CALHM1 channel, ATP leaves cell
Umami
Sensed by type II cells, stimulated by amino acids (esp glutamate & aspartate)
Detected by T1R GPCR heterodimers
promotes eating behaviour to ensure uptake of essential amino acids
Same signalling pathway as sweet based receptors:
Receptor activates--> cephalic phase insulin release --> T1R activates gustducin -->PLC activated --> release of Ca2+ from ER --> depolarisation --> non vesicular release of signaling molecule
Bitter
Chemical variety, many are toxic
Bitter sensing monomeric GPCRs (T2R)
Same pathway as sweet and umami: Receptor activates--> cephalic phase insulin release --> T2R activates gustducin -->PLC activated --> release of Ca2+ from ER --> depolarisation --> non vesicular release of signaling molecule
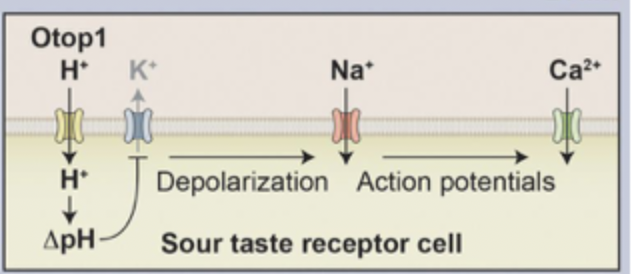
Sour
Sour sensing cells stimulated by acids, Type III taste cells have K+ leak channel
H+ ions enter cell through Otop1 channel, reduces pH (increased acidity)
K+ leak channel blocked which normally keeps cell at RMP, K+ now can’t leave cell
depolarisation bc less K+ in cell which activates VG Na+ channel, increased Na+ in cell
Release of neurotransmitter bc Ca2+ increase and synaptic vesicle fusion
Salty
Type III taste cells
ENaC(Epithelial Na+ channel) activated once it is in the membrane
Na+ increases in the cell→depolarisation → VG Ca2+ channel activated -→ synaptic vesicle fusion
Release of neurotransmitter activates gustatory afferent axon
tongue nerves
Anterior tongue= innervated by chorda tympani nerve
Posterior tongue= innervated by glossopharyngeal nerve
Taste buds off the tongue= innervated by vagus nerve
Nerve supply to the tongue= hypoglossal
Gustary input to the brain is uncrossed
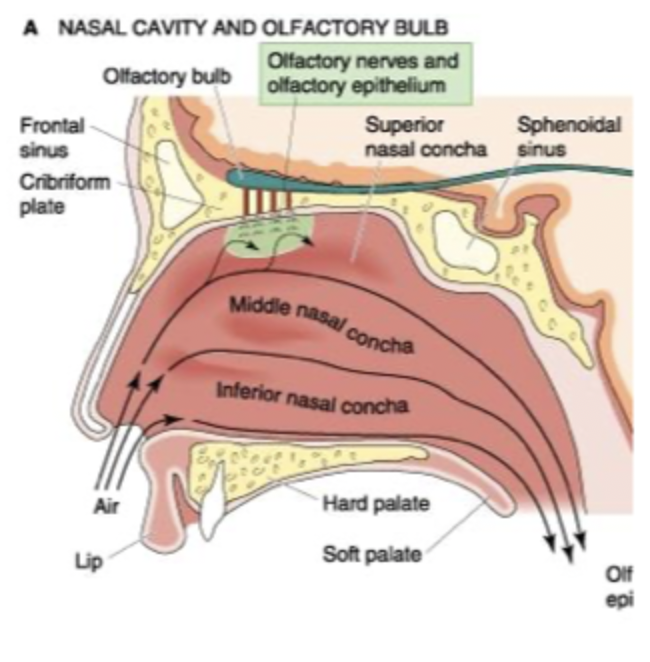
Olfactory cells, odourants, cribriform plate
inhaled and swirled around by conchae
provides a smell, dissolve in the watery mucus in roof of nasal cavity, diffuse into contact with non-motile cilia projecting from olfactory receptors, interact with receptors.
where olfactory nerves are
Anosmia, hyposmia
loss of smell
Partial loss of smell
Olfactory signal transduction
Odourant molecule binds to receptor and activates G-protein
Gs G-protein activates AC converts ATP to cAMP
cAMP activates non-specific cation channels which allow Na+ and Ca2+ to enter = depolarisation
Cl- leaves through Ca2+ activated Cl channels
further depolarisation, signal to axon hillock, reaches receptor potential
AP travels to olfactory bulb
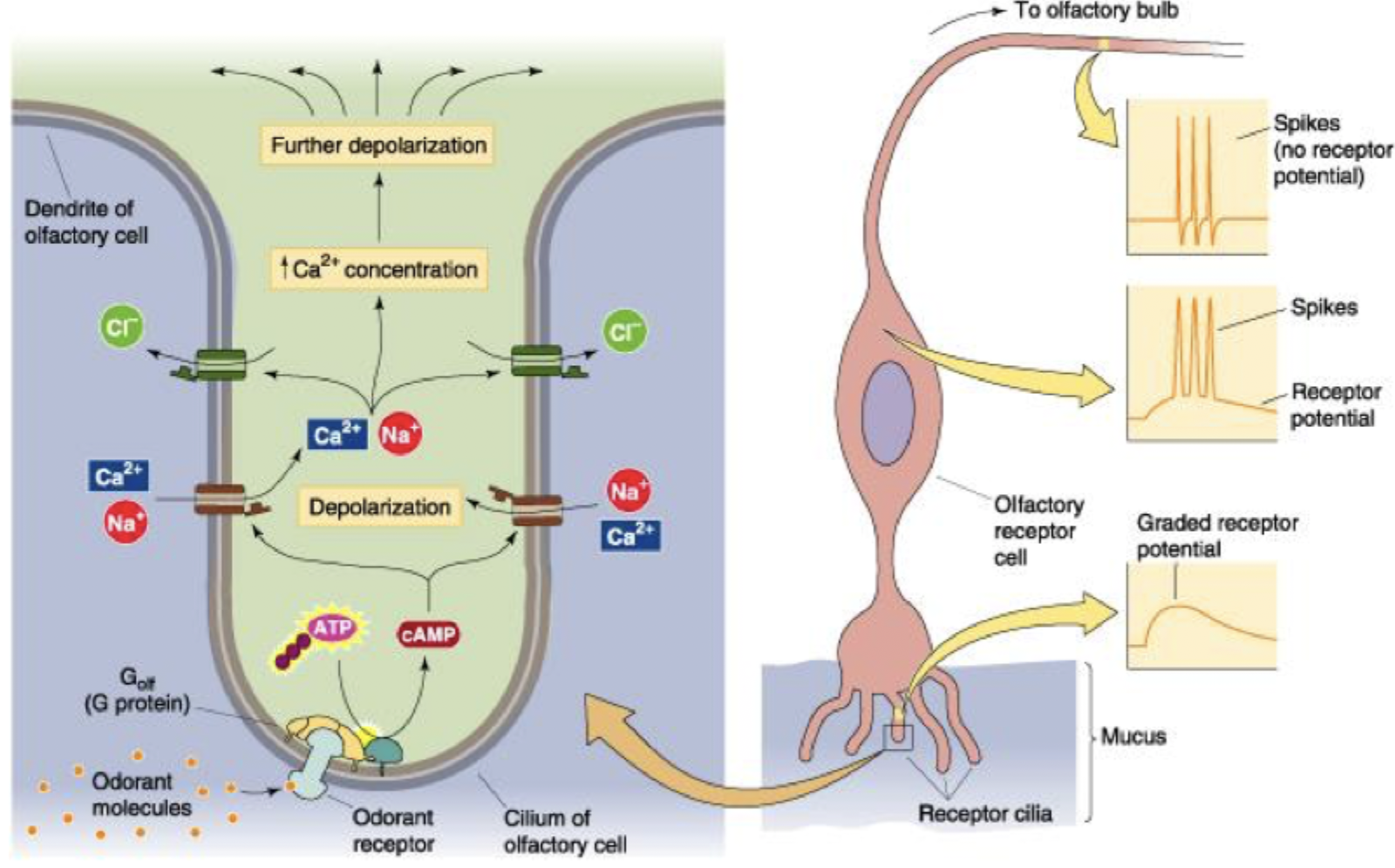
Olfactory receptor potential depolarisation, calmodulin (CAM)
AC activated, cAMP increase (ATP→cAMP), cAMP opens Na+/Ca2+ channels, cell depolarised, Ca2+ gated Cl- channels activated, further depolarisation
Calmodulin stops this signal
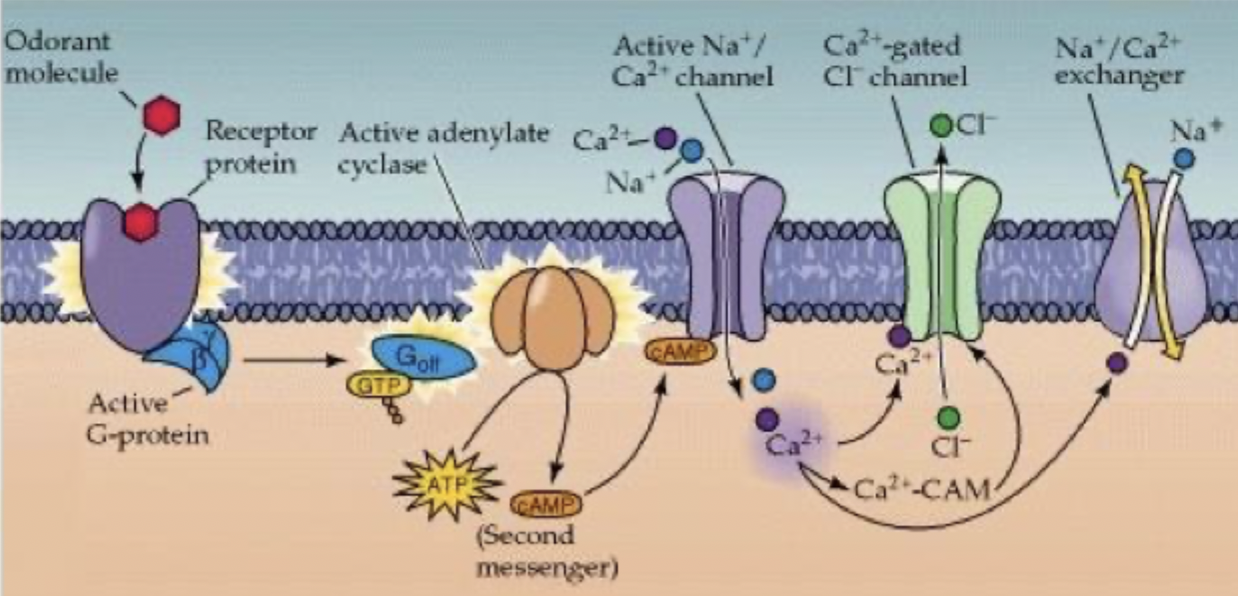
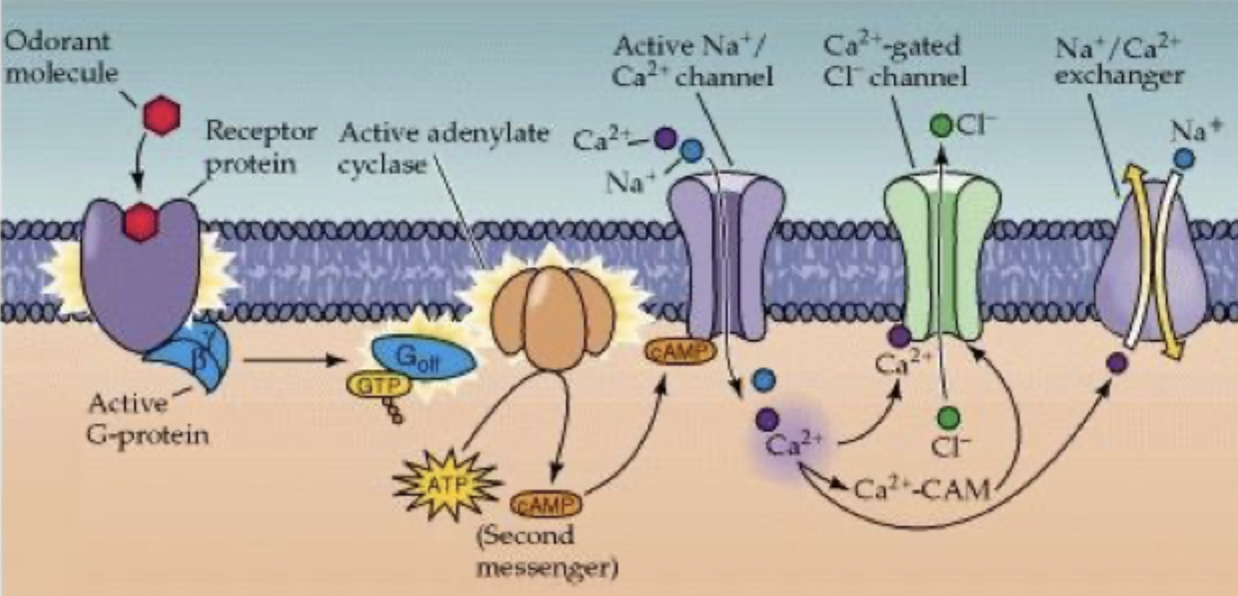
Olfactory receptor potential reduced in magnitude
cAMP broken down by phosphodiesterase
Ca2+ forms complex with calmodulin (CAM), complex binds to cAMP dependent Na+/Ca2+ channel
reduced affinity for cAMP and reduced activity of channel
Ca2+ leaves cell through Na+/Ca2+ exchanger, reduces amplitude of receptor potential
Flavor is dependent on
Input from odor receptors
Produce temperature, more energy= more smell
Product texture (e.g. bread vs toast)
Spiciness (pain)
Appearance
KCNQ2 gene encodes, what happens when it is inihibited
VG K+ channels (kV) for repolarisation phase, change in MP, inhibiting the channel increases AP firing
Ion channel that is inhibited by carbamazepine
VG Na+ channels doesn’t inhibit all of them, for seizures
Ion channel that is inhibited by alpha-conotoxin
nAChRs, at neuromuscular junction so stops muscular function
Ion channel that is inhibited by omega-conotoxin GVIA
VG Ca2+ channels, blocks synaptic transmission
Immunity to neurotoxins
immune system develops antibodies
Intrathecal injection
In spinal canal or subarachnoid space so it reaches cerebrospinal fluid.
Ziconotide is unable to cross BBB do can’t diffuse into spinal cord, reduces pain bc synaptic transmission is blocked
highest conc gets to target region= less side effects
Unmylinated vs myelinated axon velocity, loss of myelin
Small diameter, 0.5 m/s
Large diameter, 150 m/s
slower conduction velocity
Scar tissue in fovea
Dark spot in central visual field
Scar tissue on surface of optic disk, if axons are damaged
No damage
Significant damage
Scar tissue on nasal side of peripheral retina of left eye
Nasal side (ipsilateral)= doesn’t cross over so LVF
Temporal side (contralateral)= crosses over so RVF
refraction
bending of light rays as they pass from one medium to another
occurs in cornea and lens
red light
doesn’t stimulate photopigment in rods so it remains in inactive cis form.
Need to be in low light room for several mins to see well enough because
rods get photo bleached (rhodopsin is in trans form)
When you enter dark environment rhodopsin changes to inactive cis form
why does it take longer to respond after you see something
Visual events are perceived 100ms after they occur
min visual reaction time is 200ms (100ms visual detection & 100ms motor response)
need to anticipate and train to respond to fast moving objects
Large receptive fields of retinal ganglion cells
large dendritic trees, reduced visual acuity
Blind spot
at Optic disk (where optic nerve exits and blood vessels enter & exit) at nasal retina (nose side), no photoreceptors present,
When object is moved closer by your nose
eyes converge, pupil gets smaller (constriction) to keep image situated within fovea
constriction improved depth focus by using optically truest part of the eye
Changes in lens= muscle more rounded, zonule fibres relax, ciliary fibres tense
Normal vision emmytropia
far vision is normal
Lens is rounded to accomodate in near vision (zonule fibres relaxed, ciliary muscle tense)