Aldehydes ketones and Reactions
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
What is an aldehyde
Carbonyl group bonded to one carbon
suffix of Aldehyde
-anal
What is a ketone
Carbonyl group bonded to 2 carbon
Suffix of Ketone
-anone , position number
What is reduction in organic chemistry
Where a carbon forms a bond with a less electro negative element→ typically hydrogen
What are aldehydes reduced to
1 degree alcahol
Ketone reduced to
2 degree aldehydes
What reducing agent to reduce ketones and aldehydes
Sodium barohydrade NaBH(2) and Water
Mechanisim for reduction of aldehyde
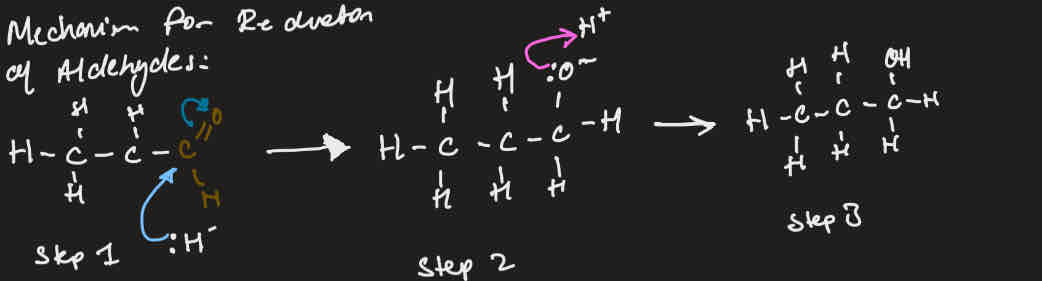
Mechanism for reduction of ketones
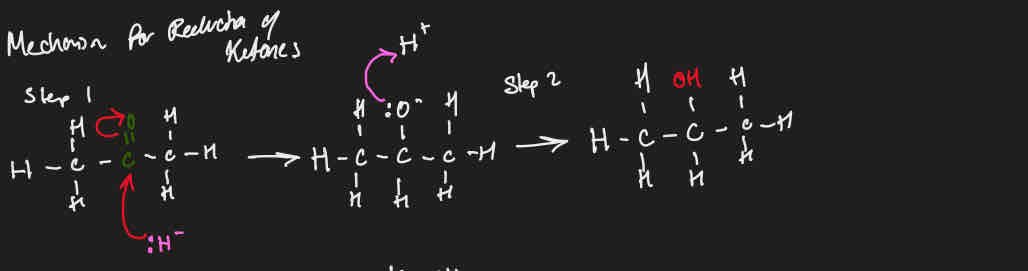
What leads to optical isomerism
Unsymmetrical ketones
What is a racemic mixture
Equal t hydrogen will bond to either side of the molecule
What leads to racemic mixture
Unsymmetrical ketones , reduced sample will lead to equal mixture of 2 enantiomers
What are enantiomers
2 compounds with exact same connectivity, that mirror images of each other