Ap Chem
1/101
Earn XP
Description and Tags
I’m gonna kms
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
102 Terms
calculate mass number
Neutrons + protons
PES (photoelectron spectroscopy)
A technique used to study the electronic structure of atoms by measuring the kinetic energy of electrons ejected from a sample when it is illuminated by ultraviolet or X-ray light.
anion
A negatively charged ion formed when an atom gains one or more electrons.
cation
A positively charged ion formed when an atom loses one or more electrons.
Higher energy levels
refer to electron shells that are farther from the nucleus, allowing for greater potential energy and less energy needed to remove and the capability to accommodate more electron repulsion, more shielding, weaker Coulumbic attraction
Higher ionization/electronegativity
More protons, greater Coulumbic attraction, greater effective nuclear charge, atomic radius decreases
Properties of ionic compounds
Compounds formed from cations and anions, characterized by high melting and boiling points, electrical conductivity when dissolved in water, and the ability to form crystalline structures.
Properties of covalent compounds
Compounds formed by the sharing of electrons between nonmetals, characterized by low melting and boiling points, poor electrical conductivity, and often existing as gases or liquids at room temperature.
Properties of network covalent
SiO2 , compounds formed by a continuous network of covalent bonds, characterized by very high melting points, hardness, and usually poor electrical conductivity.
Properties of metallic compounds
Compounds formed from metal atoms, characterized by good electrical and thermal conductivity, malleability, ductility, and a shiny luster. Sea of electrons.
Interstitial Alloy
A mixture formed when smaller atoms fit into the spaces between larger metal atoms in a metallic lattice, enhancing properties like strength and resistance to corrosion.
Substitutional Alloy
A mixture where some of the metal atoms in the metallic lattice are replaced by other metal atoms of similar size, which can alter mechanical properties such as strength and ductility.
Lattice Energy
The energy required to separate one mole of a IONIC solid into its gaseous ions, indicating the strength of the ionic bonds within the lattice. Calculated through Coulumb’s Law.
Rules of polarity
The farther away, asymmetrical, lone pairs, greater difference of electronegativity
Internuclear Distance
The distance between the nuclei of two bonded atoms in a molecule, which influences bond strength and stability.
Bond order
Total bonds/ amount of bond groups, higher=shorter + stronger, refers to the number of chemical bonds between a pair of atoms, indicating bond strength and stability.
Formal charge
The charge of an atom in a molecule, valance electrons - lines -dots. It helps assess the stability and most likely arrangement of electrons in a molecule. Only for covalent. Most electronegative element should end up with negative formal charge.
Oxidation number
The process of losing electrons or increasing oxidation state, resulting in a more positive charge. It often occurs in redox reactions, where one substance is oxidized and another is reduced. Only ionic.
Exceptions to Octet Rule
atoms with atomic numbers equal or larger than 15 can expand. Certain elements that do not strictly follow the octet rule in bonding due to their electron configurations, such as elements in the third period and beyond that can expand their valence shell, and some molecules with an odd number of electrons, leading to incomplete octets.
Resonance structure
is a way of describing the delocalization of electrons in a molecule where two or more valid Lewis structures can represent the same molecule. These structures show different arrangements of electrons but maintain the same arrangement of atoms.
2 electron domains (areas of electrons around central element)
Linear sp 180
3 domains
Trigonal planar sp2 120
3 domains, 1 lone pair
Bent sp2 <120
4 domains, 0 Lone pairs
Tetrahedral sp3 109.5
4 domains, 1 lone pair
Trigonal pyramidal sp3 <109.5
4 domains, 2 lone pairs
Bent sp3 <109.5
Lone pairs
Unshared pairs of valence electrons that influence molecular shape and an increase in polarity. Decreases degree of hybridization and bond angles.
elements that can double/triple bond
Si C N O P S
Sigma/pi bonds
Single = 1 sigma. Double = 1 sigma, 1 pi. Triple = 1 sigma, 2 pi
LDF
(London Dispersion Forces) are weak intermolecular forces arising from temporary dipoles in covalent molecules. Depends on number of electrons, and force increases when molar mass increases, increasing polarizability, and increasing the electron cloud. The greater the surface area, the greater LDF forces.
Dipole dipole
Those with smaller volumes have greater dipole dipole.interactions occur between polar molecules where positive and negative ends attract each other, causing significant intermolecular forces.
Ion dipole
WATER aka Mickey Mouse; Interactions between ions and polar molecules, where the charge of the ion is attracted to the partially charged ends of the polar molecule, influencing solubility and stability.
Hydrogen bonding
FON; is a strong type of dipole-dipole interaction that occurs when hydrogen is bonded to highly electronegative atoms like nitrogen, oxygen, or fluorine. This bond results in attraction between the hydrogen atom and lone pairs of electrons on nearby electronegative atoms, significantly affecting boiling points and solubility.
Vapor pressure/solubility
When IMF’s increase, vapor pressure decreases and vise versa/ only polar can dissolve with polar and non polar with nonpolar. Lower boiling point and higher temperature increase vapor pressure.
molar mass formula
MM=(dRT)/P
Ideal gas
High temp low presh, no IMF’s, no size. (In lower temps, molecules move slow and stick together, and in high pressure they are forced together, increasing IMF’s.
Molarity/volume given
MV=MV
Distillation
A separation process that converts a liquid mixture into its components based on differences in boiling points by heating and cooling.
Chromatography
A technique for separating components of a mixture based on their movement through a stationary phase, typically utilizing differences in affinity or solubility. Dye will go farther when matching the polarity of the solvent.
Electromagnetic Spectrum
The range of all types of electromagnetic radiation, including radio waves, microwaves(rotational), infrared(vibrational/IMF), visible light, ultraviolet(electron changes in energy levels), X-rays, and gamma rays, organized by wavelength or frequency.
Nanometer conversion (wavelength)
nm x10^-9
Beer’s law
Also known as the Beer-Lambert law, it states that the absorption of light by a solution is directly proportional to the concentration of the absorbing species and the path length of the sample. A color of a solution absorbs the complementary color (opposite in color wheel) and will spike up closest to one unit if Absorbance top determine the wavelength.
Solubility Rules
group 1, NO3, NH4
Hydroxide, nitrate, acetate, cyanide, permanganate, carbonate, sulfate, dichromate, phosphate, ammonium, chromate, peroxide, oxalate, and thiosulfate
OH-, NO3- , C2H3O2-, CN-, MnO4 - , CO3 -2 , SO4 -2 Cr2O7 -2, PO4 -3, NH4 +, CrO4 -2, O2 -2, C2O4 -2, S2O3 -2
Percent error
(Theoretical- actual)/actual. X 100
Oxidation States
O2 is -2, but is -1 on H2O2, most electronegative element should have most common charge, H2 is +1 with nonmetal and -1 with metal
Redox reactions
Chemical reactions involving the transfer of electrons, leading to changes in oxidation states. Oxidation is less electrons (on right), reduction is more electrons(on left).
Analytes
Unknown solutions
Amphoteric
substances that can act as both acids and bases.
Strong acids
HCl HBr HI HClO3 HClO4 HNO3 H2SO4
Strong bases
Li Na K Rb Cs Ca Sr Ba
Percent Yield
Actual/theoretical
Combustion
reaction that releases energy by burning hydrocarbons in the presence of oxygen, producing carbon dioxide and water.
Rate law
Can only be determined by experiment, an equation that relates the rate of a chemical reaction to the concentration of the reactants, typically expressed as rate = k[A]^m[B]^n.
Zero order
reaction rate is independent of the concentration of reactants; the rate depends only on the rate constant. Slope is -k
First order
reaction rate is directly proportional to the concentration of one reactant; as concentration increases, the rate increases accordingly. Only graph that the half life is constant in. Ln[x] slope is -k
Second order
reaction rate is proportional to the square of the concentration of one reactant or to the product of the concentrations of two reactants. The half-life is dependent on the initial concentration. 1/[x] slope is k
Catalyst/ surface area
Gets consumed, then produced. Speeds up reaction by lowering the activation energy. Greater surface area exposed, the greater the collision rate, so faster, the lower the mass, the faster it moves.
Units for k (rate constant)
M^(overall order-1)xtime^-1
Integrated rate law
is an equation that relates the concentration of reactants to time for a chemical reaction, in and given point of time. used to analyze the kinetics of the reaction.
Intermediate
Produced, then consumed. A species that is formed during a reaction and consumed in subsequent steps, not appearing in the overall reaction.
First law of thermodynamics
Energy cannot be created or destroyed, only transformed.
Enthalpy
Delta H. A measure of the total heat content of a system. It reflects the internal energy plus the product of pressure and volume, and is used to understand heat transfer in chemical reactions.
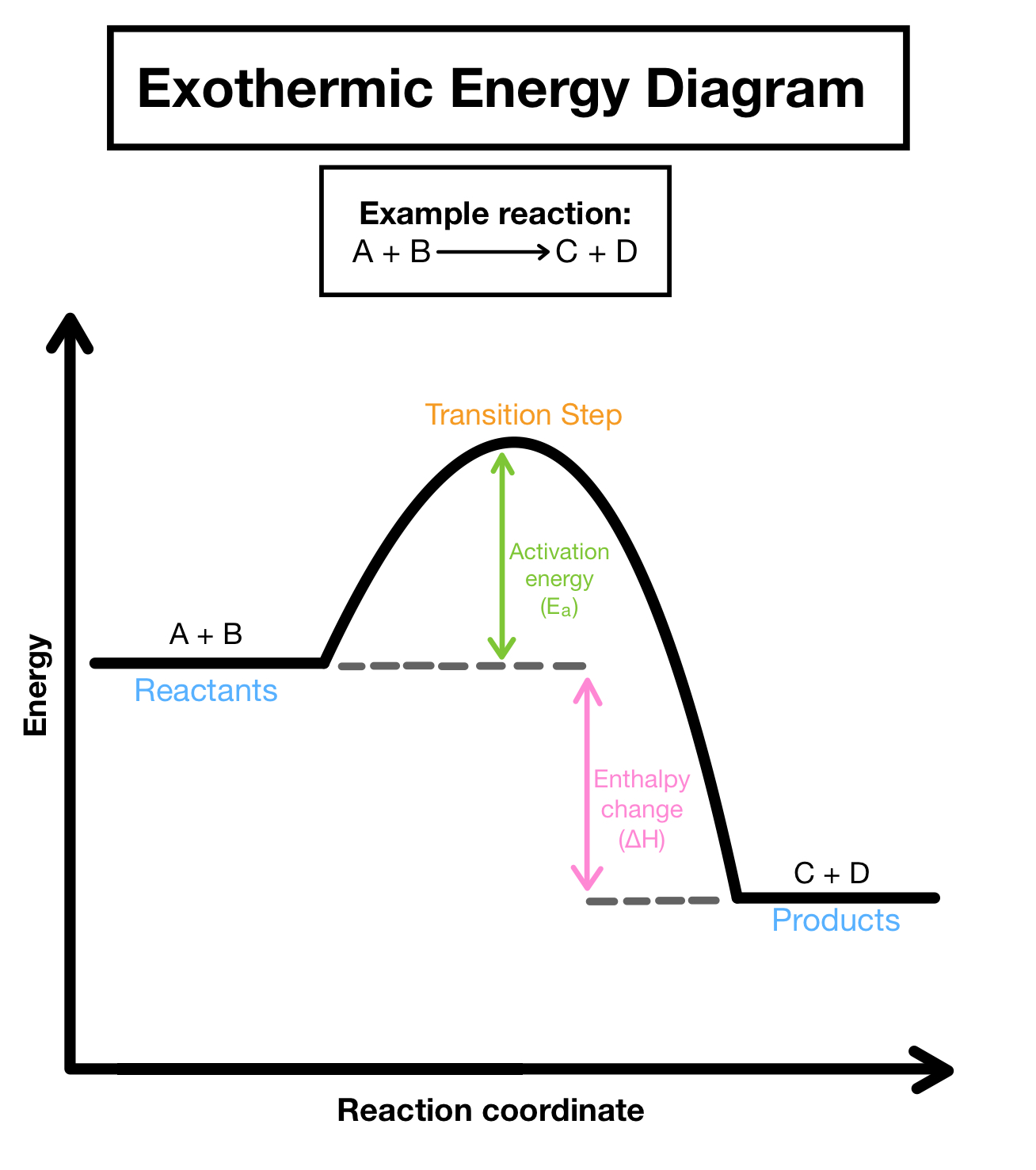
Exothermic
A process that releases heat to the surroundings, resulting in a temperature increase of the environment. Exothermic reactions typically have a negative change in enthalpy. Thermo favorable.
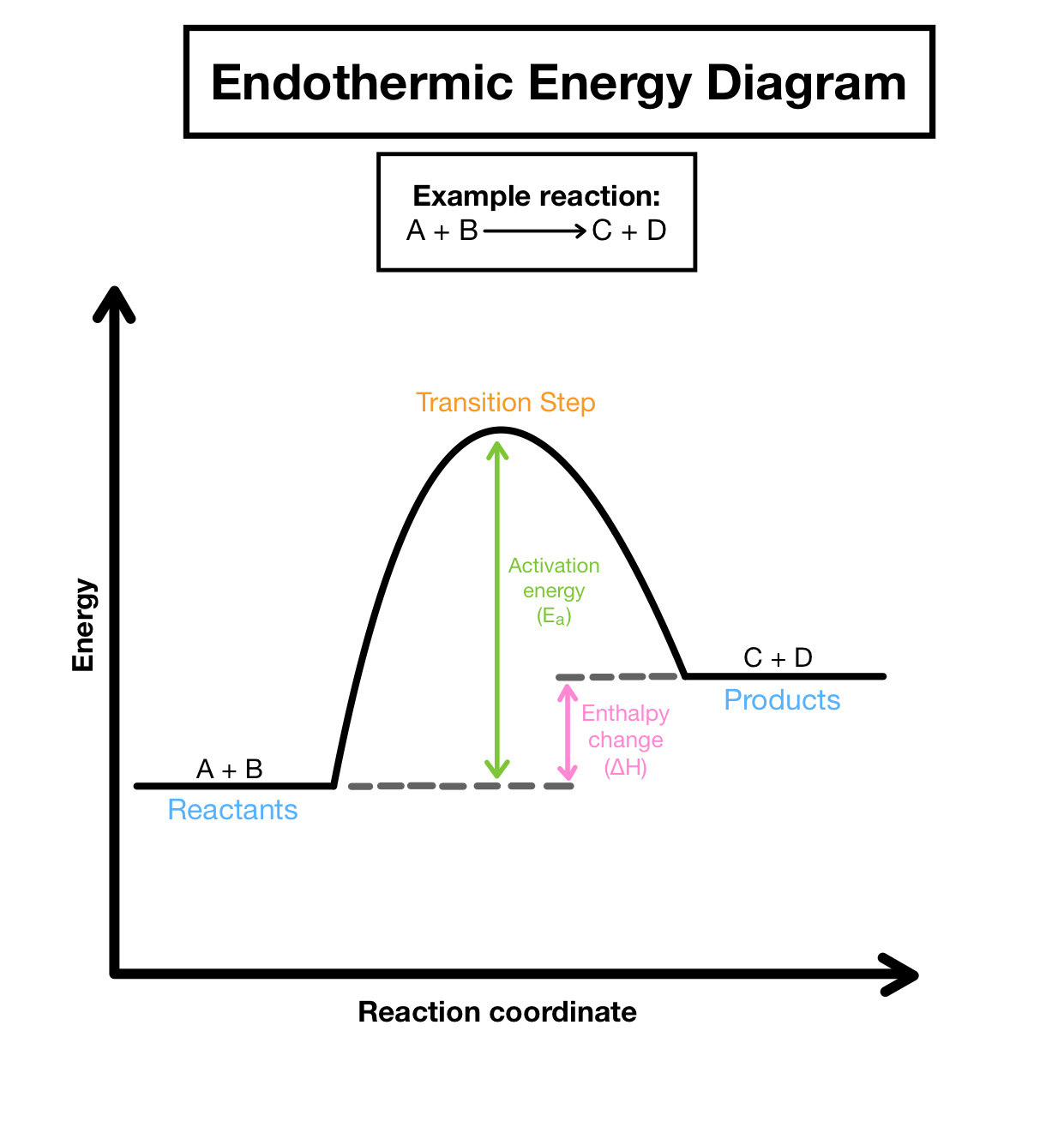
Endothermic
A process that absorbs heat from the surroundings, resulting in a temperature decrease of the environment. Endothermic reactions typically have a positive change in enthalpy.
Find delta H
Delta H = -q/mol
Bonds Thermodynamics
When bonds are given, reactants - products
Hydration energy
(ALWAYS NEGATIVE) is the energy released when ions or molecules interact with water, usually during the dissolution process. Enthalpy required to stretch out and the Enthalpy released by forming ion-dipole.
Lattice Enthalpy
If lattice>hydration it’s endothermic (more energy needed to break IMF’s) and vise versa for exothermic. The enthalpy change associated with the formation of one mole of an ionic compound from its gaseous ions. It is a measure of the strength of the forces between the ions in an ionic solid.
Phase changes
Temperature remains constant. Enthalpy of fusion(melting), vaporization, and sublimation (solid to gas) ALL endothermic, if other way, exo. H vaporization > H fusion
Specific heat ( c )
Larger specific heat= lower temp change, reciprocal of slope in melting-boiling graph
Energy/heat released (q)
The total amount of energy or heat released or absorbed during a physical or chemical process, often measured in joules or calories. add water for mass if solution dissolves
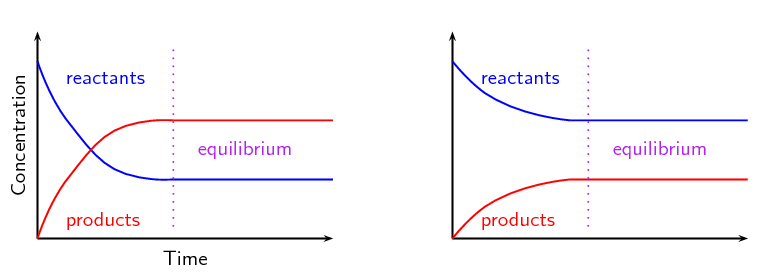
Equilibrium graph
The reactant/product would go up twice as much if 2 to 1 ratio
Equilibrium constants
NO SOLIDS OR LIQUIDS. A numerical value that expresses the ratio of the concentration of products to reactants at equilibrium, raised to the power of their coefficients in a balanced equation.
K and Q
K<1 reactants favored, K>1 products favored. K=1 is equilibrium. K<Q need to make more reactants, reverse reaction K>Q need to make more products, forward. K will ONLY change if temperature changes, Q will ONLY changes with concentration, pressure, and temperature. Refers to the equilibrium constant (K) and the reaction quotient (Q)
Manipulating K and H
K - flipping is reciprocal, multiplying is exponent, when adding all K values, multiply
H - flipping is changing sign, multiplying is multiplying, when adding all H values, add
Le Chateliers Principle
More products = shift left, more reactants = shift right, when volume decreases and pressure increases, shift to fewer moles. When volume increases and pressure decreases, shift to more moles. New gas introduced when volume increases/decreases, total pressure stays the same but partial pressure increases, shift to more/fewer moles. When new gas is added but volume is constant, only total pressure increases, no shift.
Dilution
Shifts to the side with more aqueous species. The process of reducing the concentration of a solute in a solution, typically by adding more solvent. Dilution affects the concentration of a solution while the total amount of solute remains constant.
Temperature shifts
Exo= increase in temp means shift left and decreases K Endo=increase in temp means shift right and increases K
Solubility product (Ksp)/saturation/molar solubility
It expresses the extent to which a compound can dissolve in solution. (Only use when double arrows) K>Q more solid will dissolve and K<Q solid will precipitate K=Q is saturated.SATURATED= no more solute can dissolve. MOLAR SOLUBILITY= amount of solid that can dissolve to make 1 Liter. (Increases with rising temperature) M.S WILL BE EQUAL TO ALL SUBSTANCES IF 1 TO 1 RATIO (strong acids will split, weak stay)
Common ion effect
The phenomenon where the solubility of a salt is REDUCED in the presence of a common ion, resulting in a decrease in the concentration of the dissolved ions. Will shift to left. SALT WITH LESS KSP WILL PRECIPITATE FIRST
Strong Acids/Bases components
All concentrations are equal (remember mole ratio) Strong acids and bases dissolve more and higher Ka/b values and lower pKa/b values. (THE LISTED STRONGEST ACIDS/BASES DONT HAVE Ka/b OR PKa/b) They have weaker conjugate bases/acids. They are less stable and more reactive so they are less favored in equilibrium, so the conjugates are more favored. No double arrows. More electronegative means stronger acid
pH
Greater concentration of H+ means lower pH means more soluble, more acidic, greater concentration of OH- means lower pOH (higher pH), less soluble, more basic
Weak Acids/bases components
Usually Ka=x²/number-x(which you assume =x) Do not completely dissociate in solution, resulting in a mixture of undissociated acid/base and its ions. They have higher pKa/b values compared to strong acids/bases and exhibit equilibrium with their conjugate counterparts.
Percent Dissociation
[H+]/[acid] is the ratio of the concentration of dissociated acid or base to its initial concentration, expressed as a percentage. It indicates the strength of weak acids or bases in solution.
Autoionization of water
Kw, Endothermic, when temperature increases, concentration of H and OH increase, and lowers both pH and pOH. Increasing pH decreases [H]
Polyprotic acids
[H3PO4]>[H2PO4-]>[HPO4-2]Acids that can donate more than one proton (H+) per molecule, resulting in multiple dissociation steps and corresponding equilibrium constants. Examples include sulfuric acid and phosphoric acid.
![<p>[H3PO4]>[H2PO4-]>[HPO4-2]Acids that can donate more than one proton (H+) per molecule, resulting in multiple dissociation steps and corresponding equilibrium constants. Examples include sulfuric acid and phosphoric acid. </p>](https://knowt-user-attachments.s3.amazonaws.com/faa8e078-44b6-49c1-b175-27806e58e3c2.png)
Strong acid + strong base
H+ + OH- = H2O
Strong acid + weak base
H+ + weak base = CA
Weak acid + strong base
Weak acid + OH- = CA + H2O
Weak acid + weak base
Weak acid + weak base = CA + CB
Buffer
A solution that resists changes in pH upon the addition of small amounts of acid or base, typically composed of a weak acid and its conjugate base. Strong acids or bases can’t be used because [H] will remain constant. The higher the concentration of the conjugate pair, the more effective the buffer will be. If a buffer has more CA then more effective when base gets added, but if higher CB then more effective when acid is added.
Buffer for base equation
pOH=pKb + log [CA]/[base]
Indicators
weak acids that change color in response to pH changes, used to determine the endpoint of titrations.
pKa (half-equivalence point)
The pH at which the concentrations of an acid and its conjugate base are equal, indicating that half of the acid has been neutralized during a titration. pH>pKa more weak acid, pH decreases. pH<pKa more CB pH decreases.
Equivalence point
Moles of base = moles of acid, The point in a titration when the amount of titrant added is stoichiometrically equivalent to the amount of substance being titrated, resulting in a complete reaction. Acid and bases are not present, only conjugates remain in solution afterward.
Entropy
Less moles to more moles is positive(ALL HAVE TO BE THE SAME PHASE CHANGE). And solid to gas is positive. CONVERT J TO KJ. If volume increases, S increases bc more dispersion. A measure of the disorder or randomness in a system, often associated with the number of ways a system can be arranged. In thermodynamics, it is a key factor in determining the spontaneity of processes.
Gibbs free energy
Delta G. Negative is favorable, positive is unfavorable. Delta G=0 is equilibrium. TO FIND BOILING POINT ASSUME G IS 0. A thermodynamic potential that measures the maximum reversible work obtainable from a system at constant temperature and pressure. It combines enthalpy and entropy to determine the spontaneity of a process.
Standard Reduction Potentials
FLIP SIGN TO GET OXIDATION POTENTIAL. so, the lower reduction, the higher the oxidation. IF METAL GETS OUT IN SOLUTION AND SOLID DOESN’T FORM, THE REDUCTION POTENTIAL OF METAL IS HIGHER THAN SOLUTION AND VICE VERSA. A measure of the tendency of a chemical species to acquire electrons and be reduced, typically expressed in volts.
Galvanic Cell
Has salt bridge that gives anions to anions and cathodes to cathodes. Anode gives electrons off to cathode. At the cathode, solid gets made.A device that converts chemical energy into electrical energy through spontaneous redox reactions. It consists of two separate half-cells connected by a wire and an electrolyte.