6. Bond polarity + electronegativity
1/6
Earn XP
Description and Tags
page 5 on packet
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
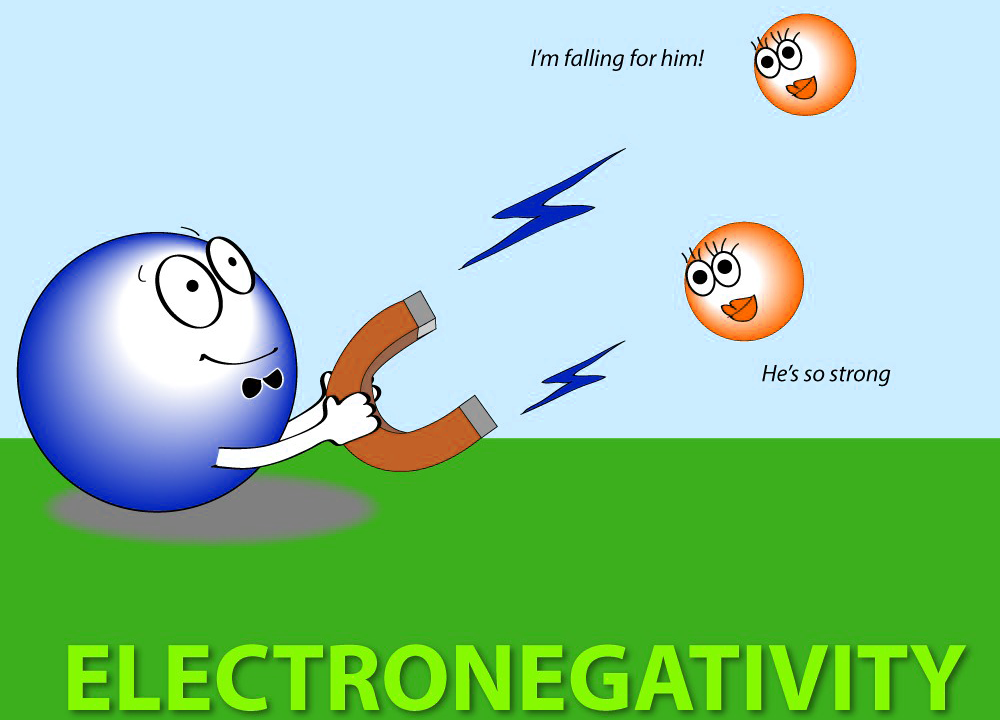
What is electronegativity?
The ability of an atom in a covalent bond to attract shared electrons.
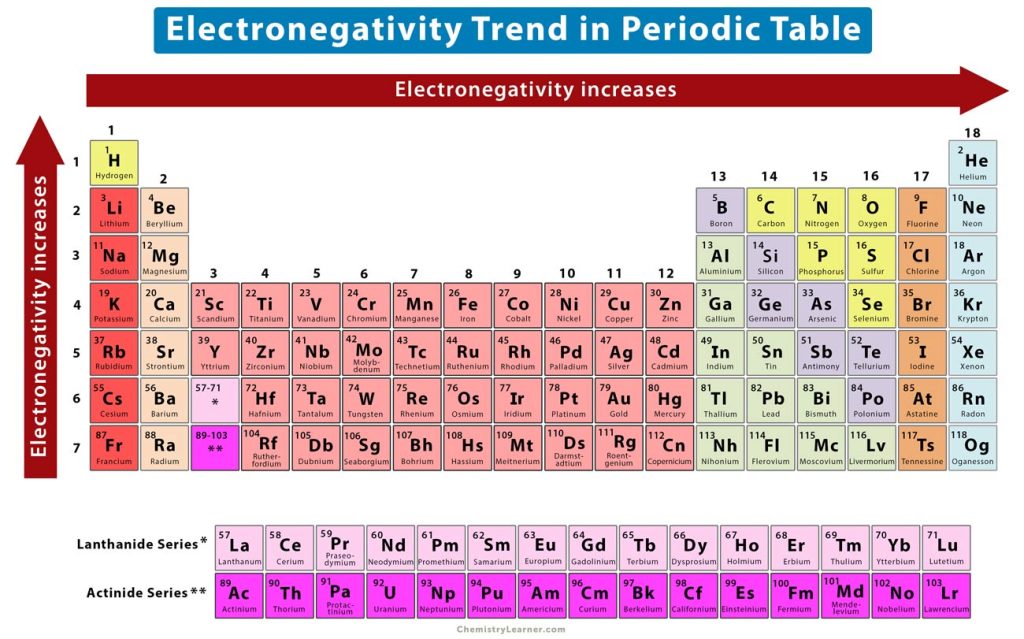
What is the electronegativity trend?
F>O>N>Cl>Br (up into the right)
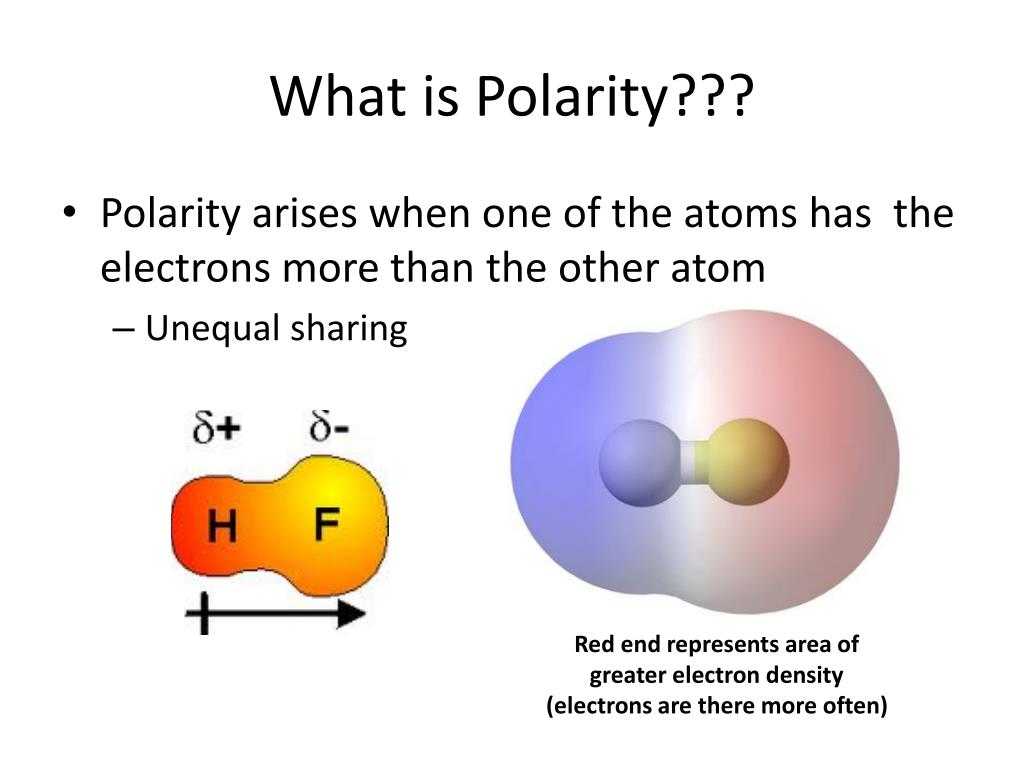
What does polar mean?
Something that has an uneven distribution of electrical charge (one side is positive, one is negative)
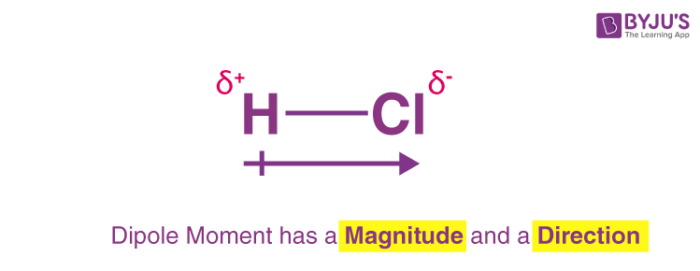
What is a dipole moment?
electrons are shared unevenly in a compound, chemists use an arrow to show the direction in which the electrons are being pulled (mu unit)
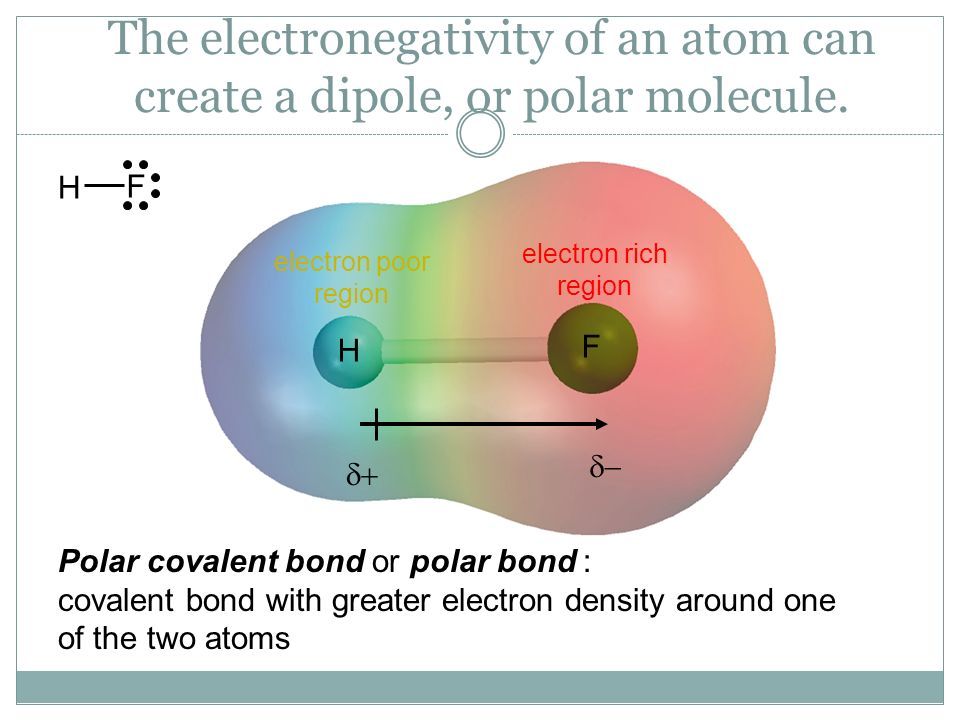
What is a polar-covalent bond?
Covalent bond in which electrons are not shared equally, resulting in a net dipole
moment (μ)
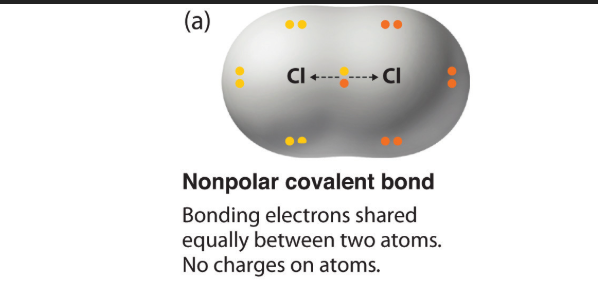
What is a NON-polar covalent bond?
Covalent bond in which electrons are shared relatively equally

What is a ionic bond?
when electrons transfer from one atom to another