Biochemistry I Module of the MCAT Self Prep eCourse: Lesson 12: Carbohydrates (Pro)
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
Lesson 12: Carbohydrates
Lesson 12: Carbohydrates
Describe how the name "carbohydrate" relates to the structure of a carbohydrate.
The prefix "carbo-" and the suffix "hydrate" refers to the fact that carbohydrates contain carbon and water in general formula: Cn(H2O)n
CRB Based on the general structure of a Carbohydrate, you can expect lots of Oxygen-containing functional groups. Which of the following would you LEAST expect to see in a carbohydrate?
(A) Carbonyl
(B) Ether
(C) Carboxylic Acid
(D) Alcohol
(C) Carboxylic Acid
Carbonyls, ethers, and alcohols are all commonly seen in carbohydrates.
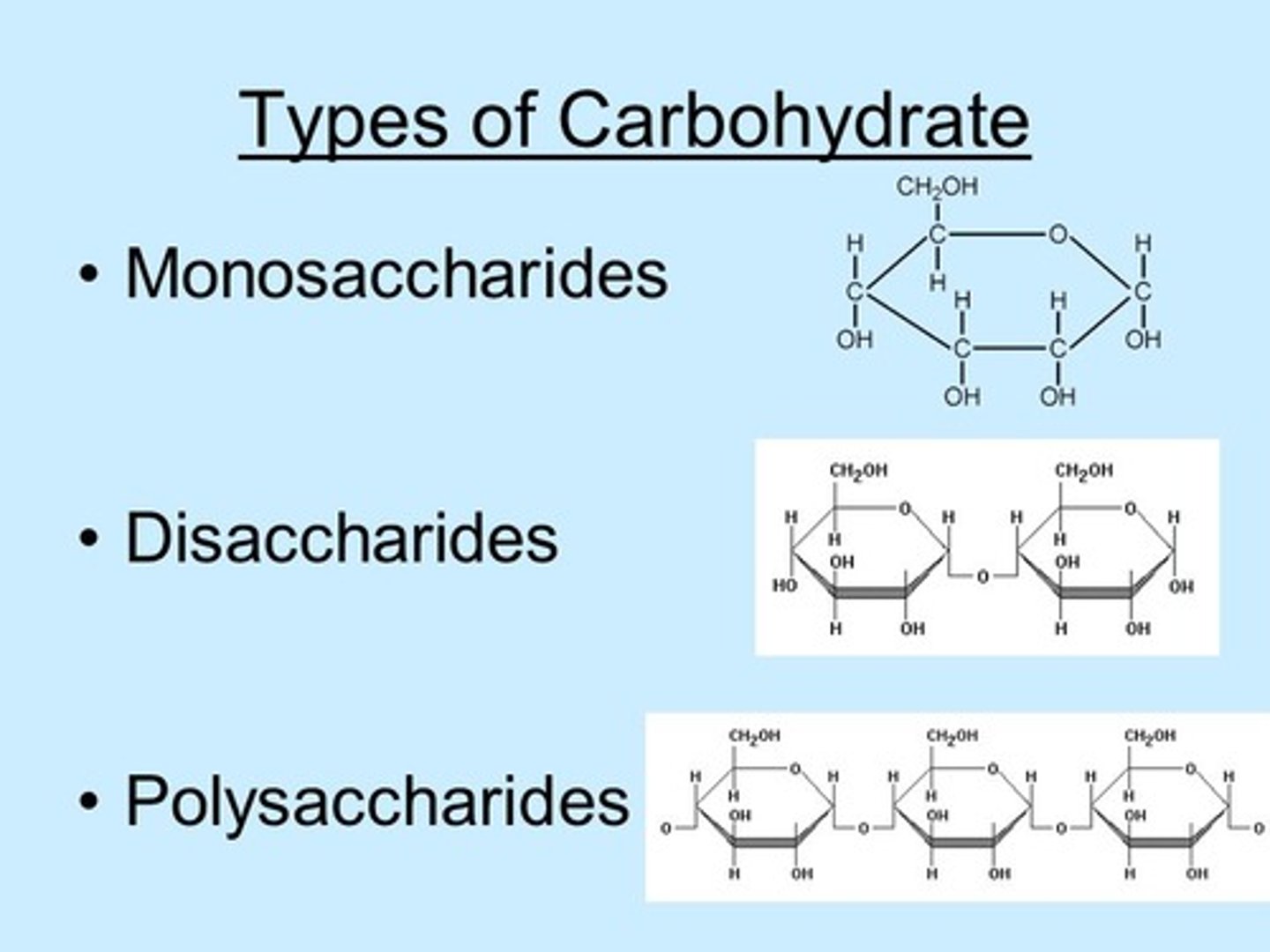
What is the difference between monosaccharides, disaccharides, and polysaccharides?
Monosaccharides consist of a single sugar monomer.
Dissaccharides consist of two sugar monomers linked together.
Polysaccharides consist of three or more sugar monomers linked together.
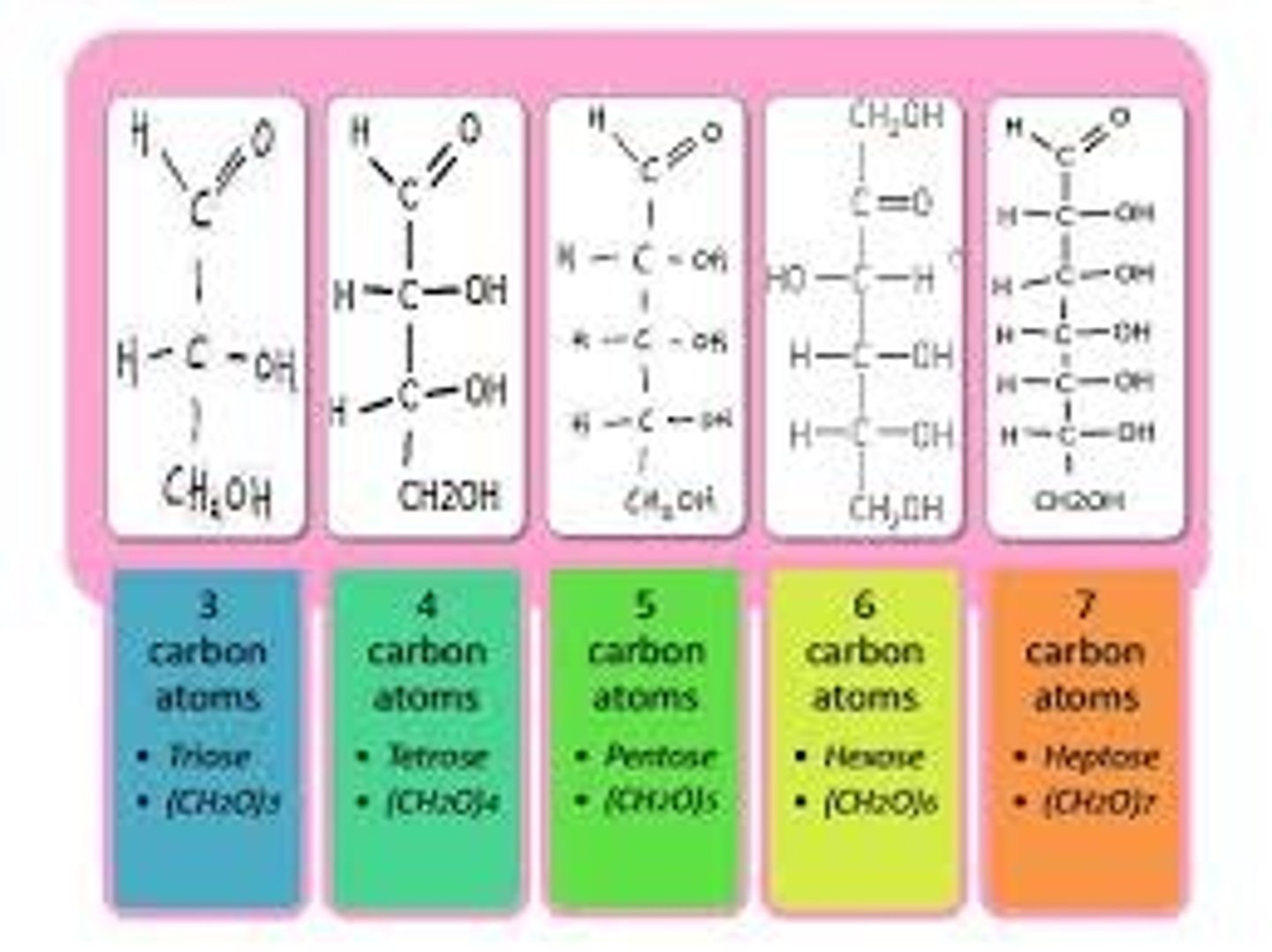
What is the difference between trioses, tetroses, pentoses, and hexoses?
Trioses are sugar monomers made up of three carbons.
Tetroses are sugar monomers made up of four carbons.
Pentoses are sugar monomers made up of five carbons.
Hexoses are sugar monomers made up of six carbons.
CRB True or false? Trioses are the simplest of all monosaccharides.
True. Trioses are the simplest of all monosaccharides.
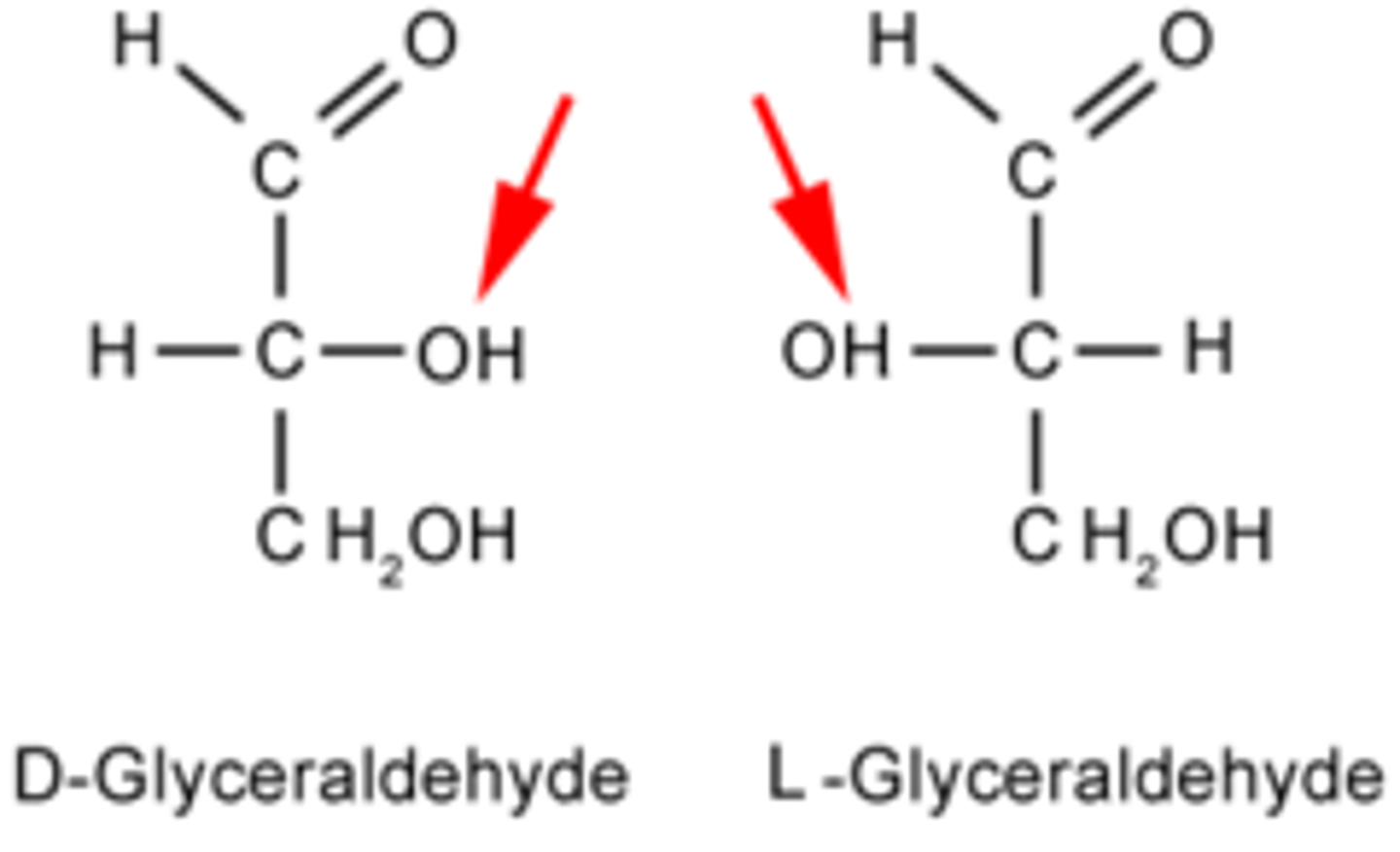
Draw or visualize a D- versus L-glyceraldehyde. What makes a sugar monomer D versus L?
When drawn as Fischer projections,
D sugar monomers have the OH group on the right for the final stereocenter.
L sugar monomers have the OH group on the left for the final stereocenter.
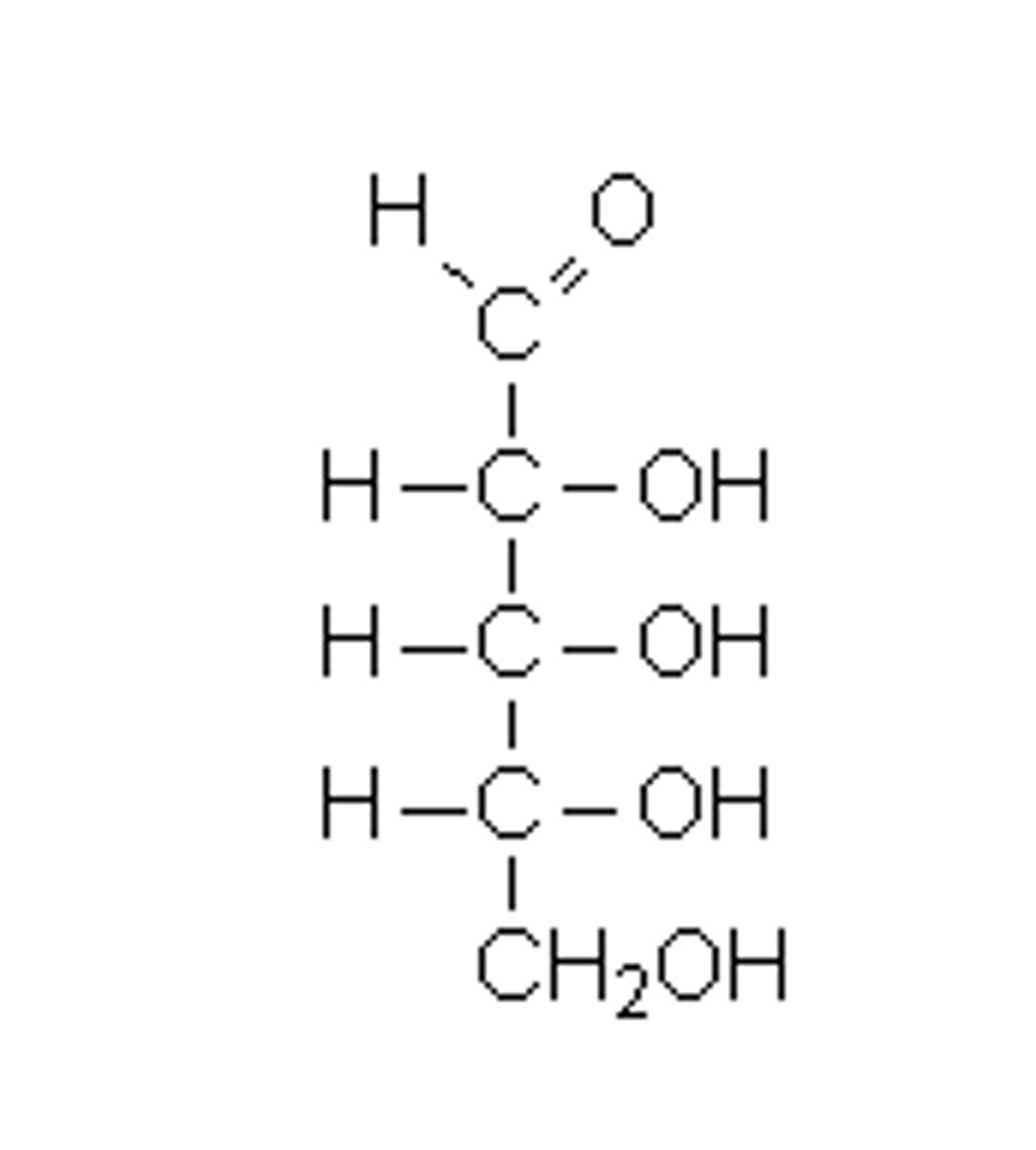
Draw a D-aldopentose carbohydrate.
An aldopentose has 5 carbons, which gives it the suffix "pentose." It contains an aldehyde functional group, which gives it the prefix "aldo-". Lastly, the OH is located on the right of the last stereocenter carbon giving it a D configuration.
Draw a D-ketohexose carbohydrate.
A ketohexose has 6 carbons, which gives it the suffix "hexose." It contains an ketone, which gives it the prefix "keto-". Lastly, the OH is located on the right of the last stereocenter carbon giving it a D configuration.
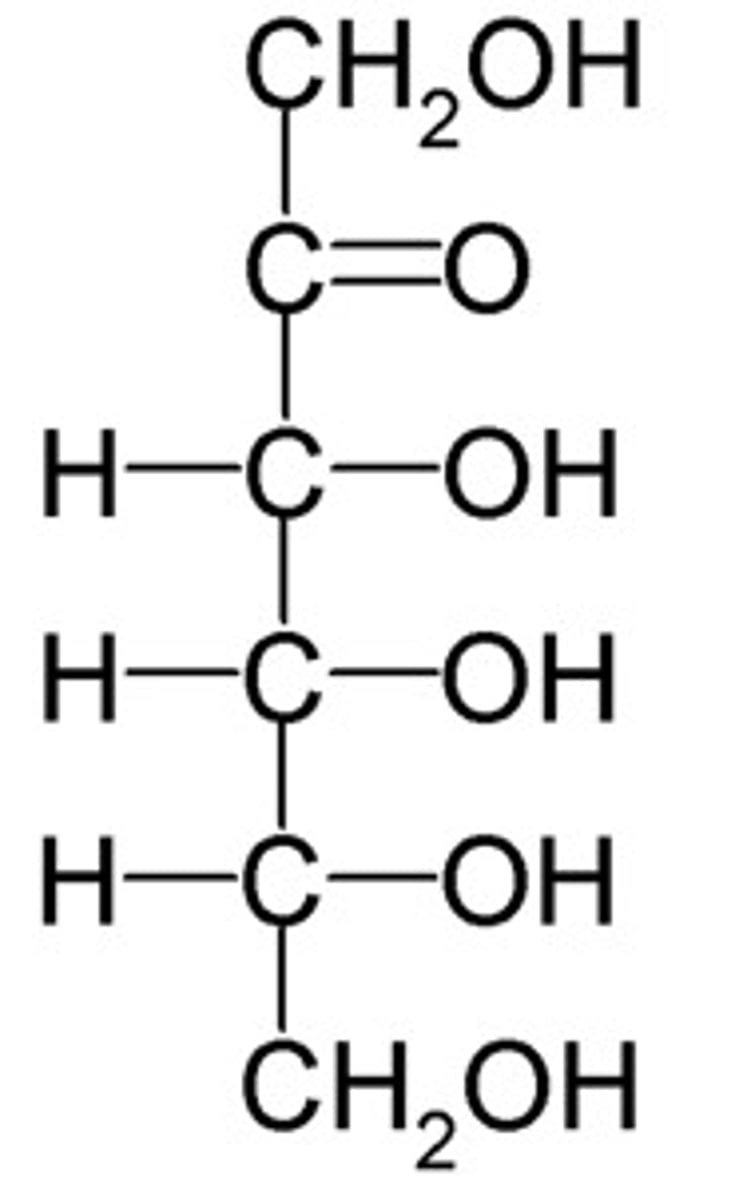
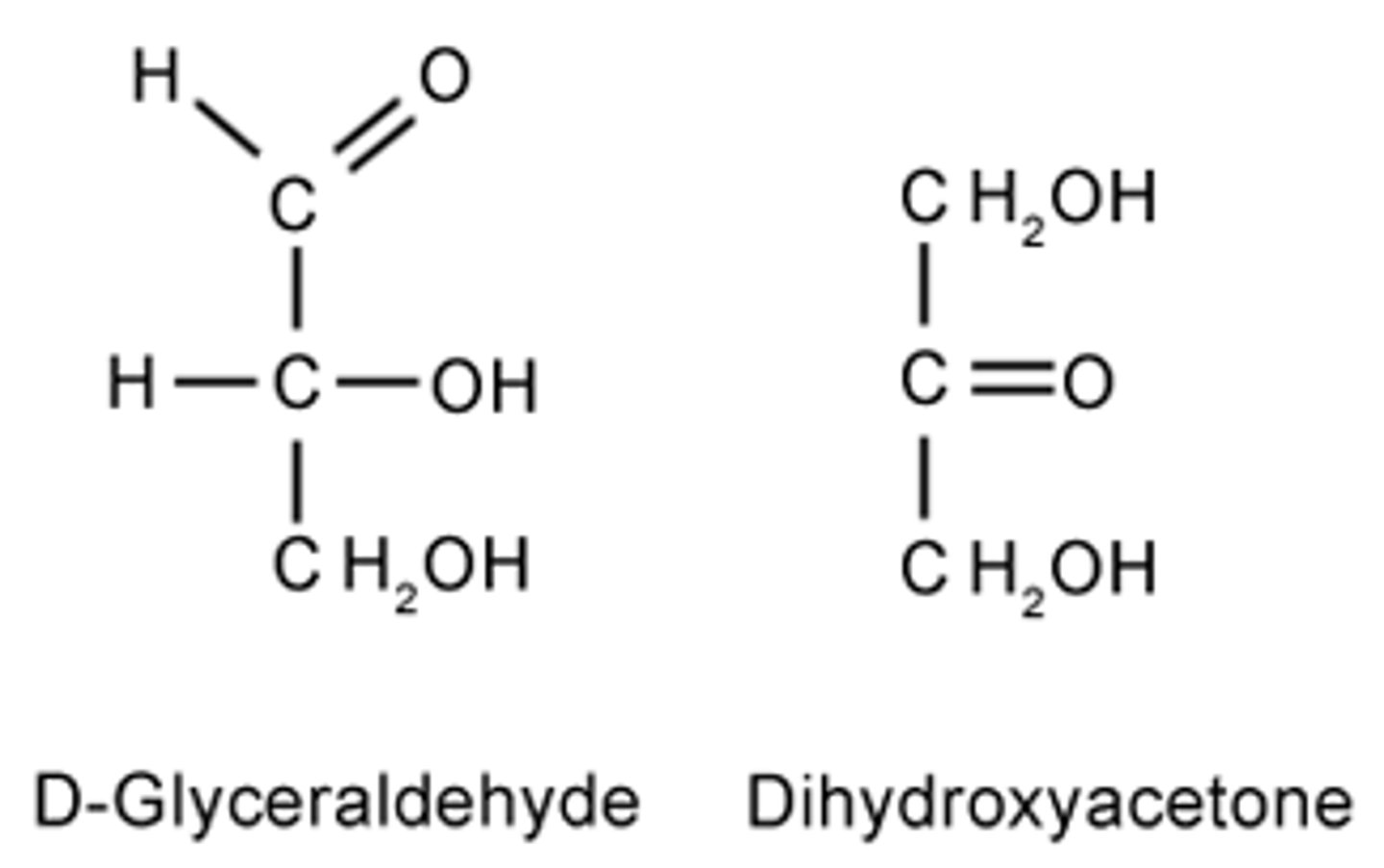
CRB Fill in the blanks: The simplest Aldose is ____________, whereas the simplest Ketose is ______________.
(A) Glyceraldehyde, Ketohexose
(B) Glyceraldehyde, Dihydroxyacetone
(C) Aldopentose, Dihydroxyacetone
(D) Aldopentose, Ketohexose
(B) Glyceraldehyde, Dihydroxyacetone
The simplest Aldose is Glyceraldehyde, whereas the simplest Ketose is Dihydroxyacetone.
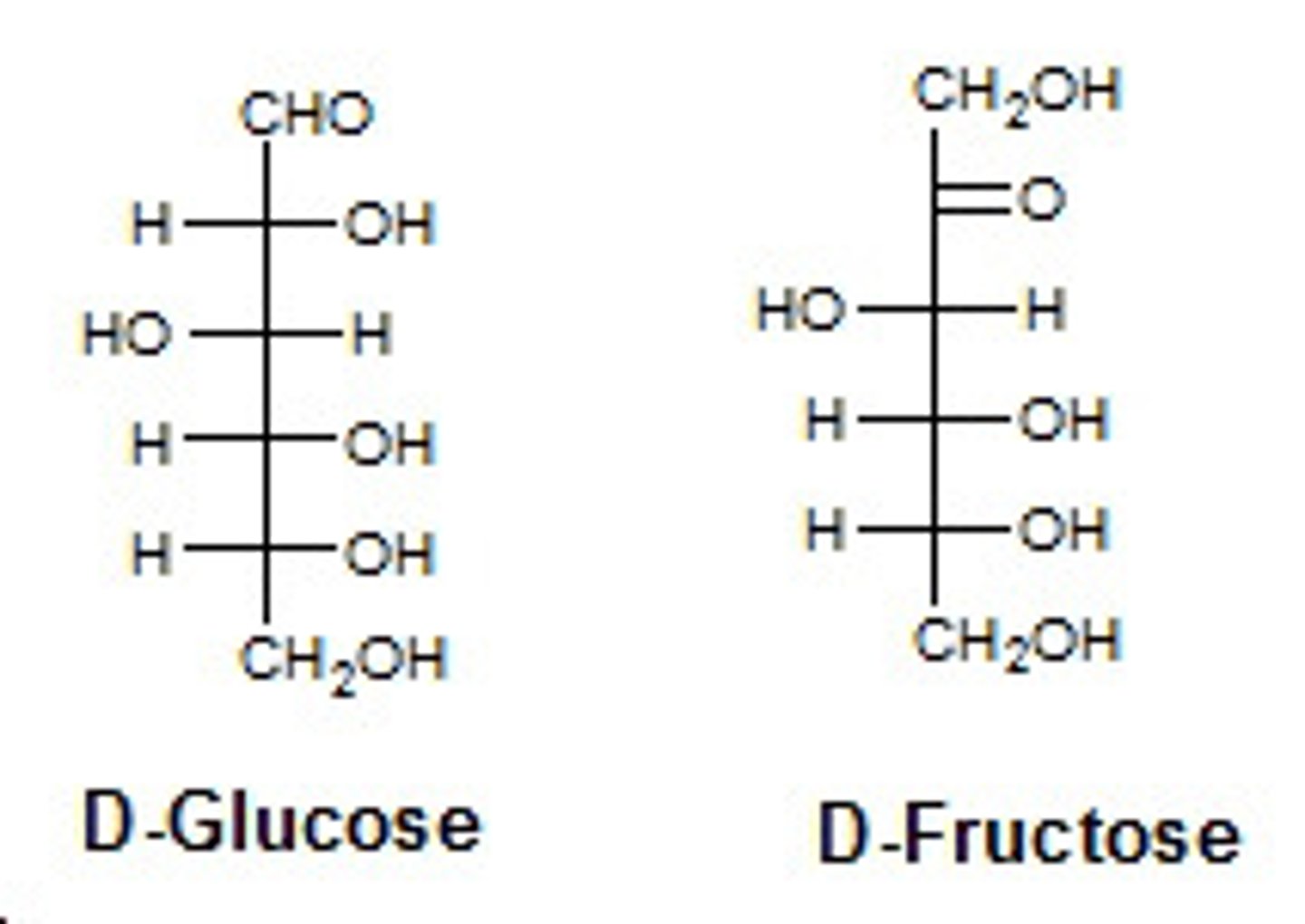
Draw or visualize D-glucose versus D-fructose. These two compounds are considered:
(A) diasteriomers
(B) epimers
(C) constitutional isomers
(D) enantiomers
(C) constitutional isomers
These two compounds are considered constitutional isomers because they have the same molecular formula with different connectivity.
D-glucose is an aldose, while D-fructose is a ketose.
What are diasteriomers versus epimers?
Diastereomers are stereoisomers with a different configuration (R /S) at one or more (BUT NOT ALL) of the chiral carbons.
Epimers are a type of diastereomers that differ at only one carbon configuration.
Which of the following statement(s) is true of a diastereomers?
I. They have similar physical properties
II. They have different physical properties
III. They have similar chemical/biological properties
IV. They have different chemical/biological properties
(A) I and III Only
(B) II and IV Only
(C) I Only
(D) III Only
(B) II and IV Only
Diastereomers are molecules that are different from each other in terms of their physical and chemical/biological properties.
True or False? A sugar monomer in the D configuration will rotate plane-polarized light to the right.
False. Sugar monomers in the D configuration will sometimes rotate plane polarized light to the right and sometimes rotate it to the left. The D/L configuration has no correlation to the direction the compound will rotate plane polarized light.
Compare the R/S, +/-, d/l, and D/L stereochemistry designations.
R/S designations are given to single stereocenters based on whether or not their substituents go from highest to lowest priority group in the clockwise (R) or counterclockwise (S) direction.
d (aka +)/l (aka -) designations are given to compounds as a whole depending on whether they rotate plane-polarized light to the right (d/+) or to the left (l/-).
D/L designations are given to sugar monomers as a whole. The compound is considered D if its final stereocenter has the R configuration, and is considered L if its final stereocenter has the S configuration.
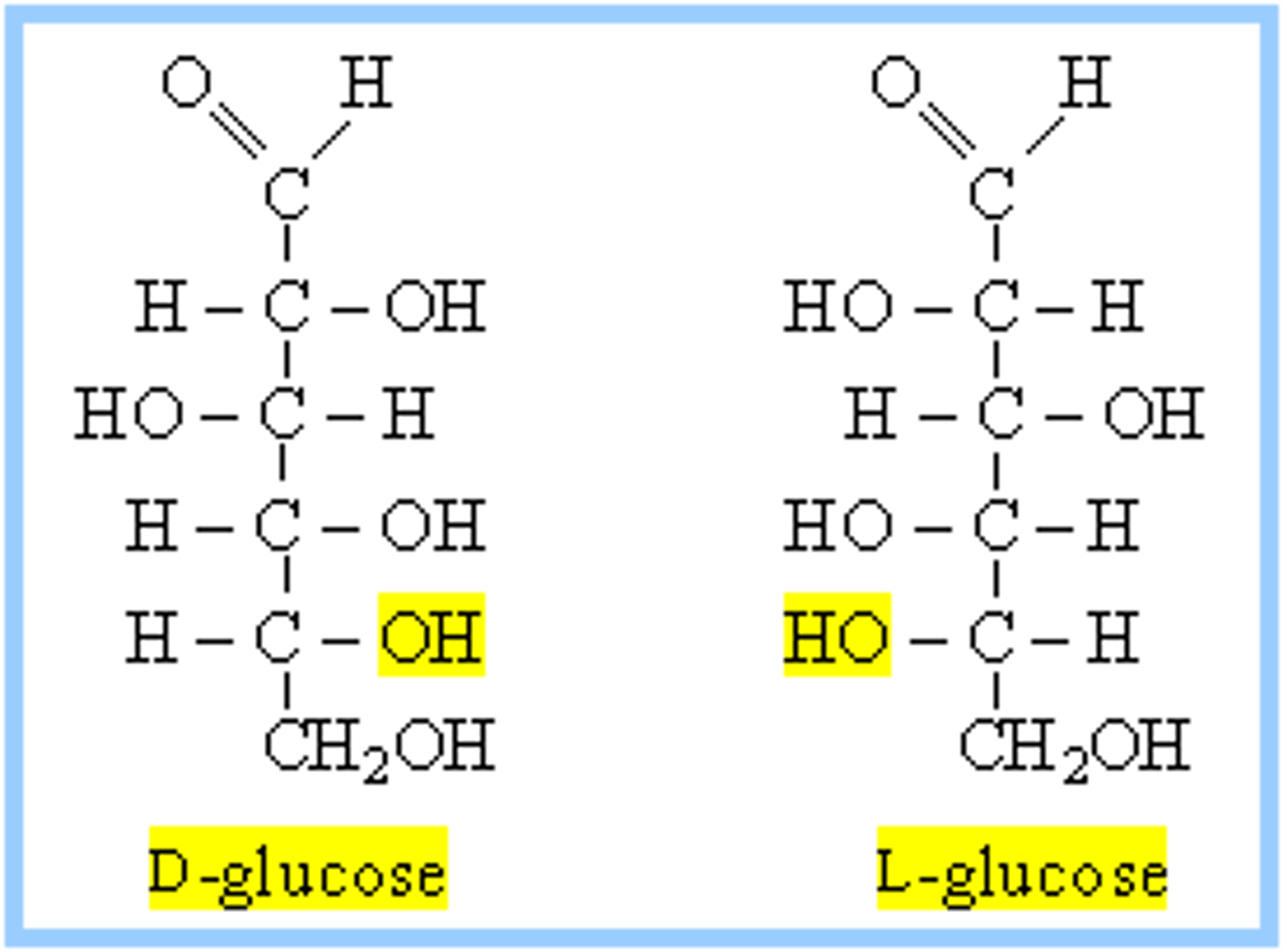
Draw or visualize D- versus L-glucose. These two compounds are considered:
(A) diasteriomers
(B) epimers
(C) constitutional isomers
(D) enantiomers
(D) enantiomers
These two compounds are considered enantiomers because they have the opposite stereochemistry designation (R/S) at every stereocenter.
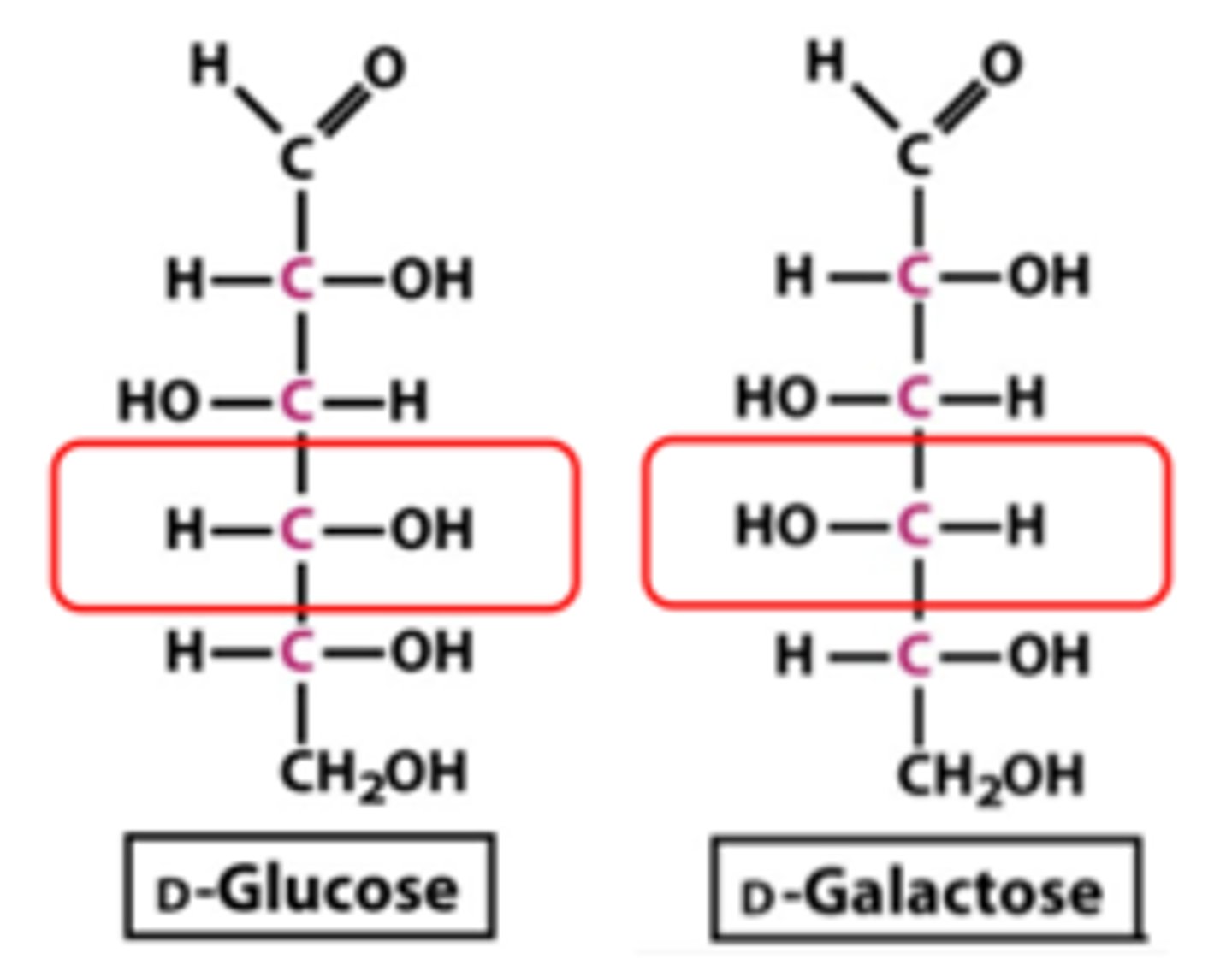
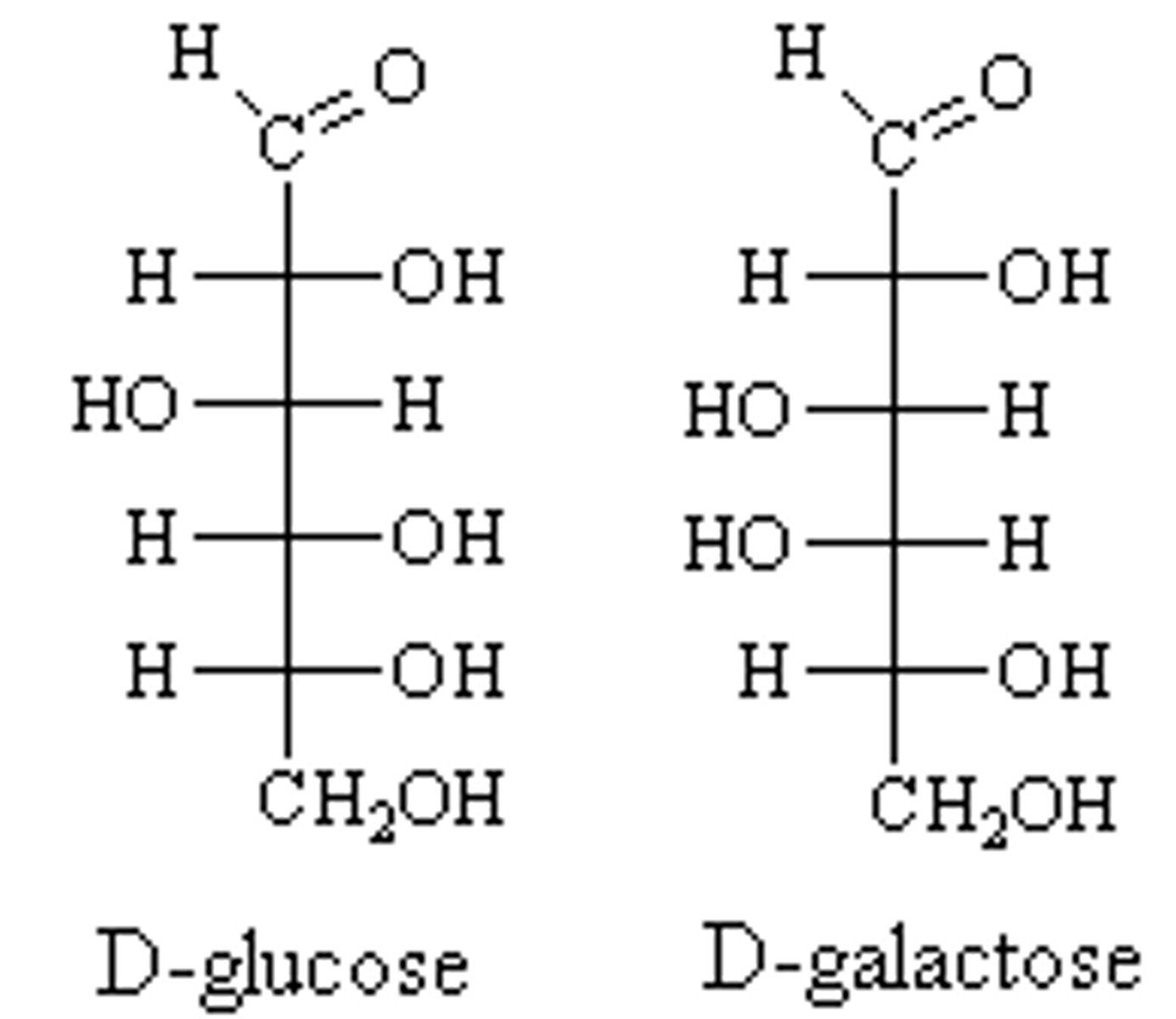
Draw or visualize D-glucose versus D-galactose. These two compounds are considered:
(A) diasteriomers
(B) epimers
(C) constitutional isomers
(D) enantiomers
(B) epimers
These two compounds are considered epimers because they have the opposite stereochemistry designation (R/S) at a single stereocenter.
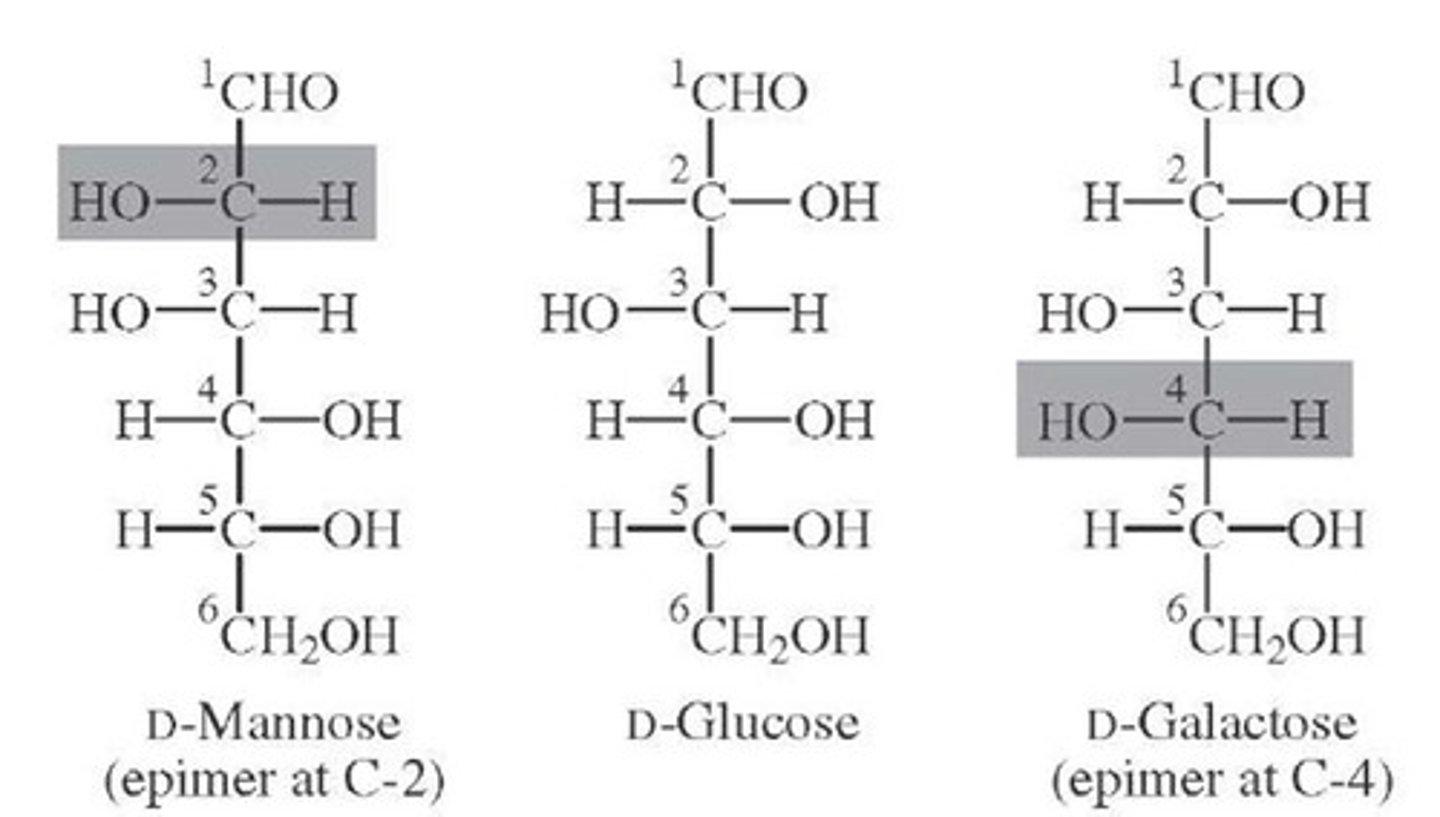
Draw or visualize D-glucose versus D-mannose. These two compounds are considered:
(A) diasteriomers
(B) epimers
(C) constitutional isomers
(D) enantiomers
(B) epimers
These two compounds are considered epimers because they have the opposite stereochemistry designation (R/S) at a single stereocenter.
How many possible D-aldohexoses are there?
(A) 4
(B) 6
(C) 8
(D) 16
(C) 8
An aldohexose has 4 chiral centers. You can calculate the possible number of configurations using the formula 2^n where n is the number of stereocenters. 2^4 is 16, so there are 16 possible aldohexoses (8 D-aldohexoses and 8 L-aldohexoses).
CRB Fill in the blank: Knowing that any specific Aldohexose has 4 chiral centers, it will have __________ enantiomer(s) and __________ diastereomer(s).
(A) 2 , 5
(B) 2 , 14
(C) 1 , 6
(D) 1 , 14
(D) 1 , 14
Knowing that Aldohexoses have 4 chiral centers, they can have 1 enantiomer and 14 diastereomer(s).
Note that any molecule can only have one Enantiomer, and that one of the 16 possible Aldohexoses is the original Aldohexose everything else is being compared to.
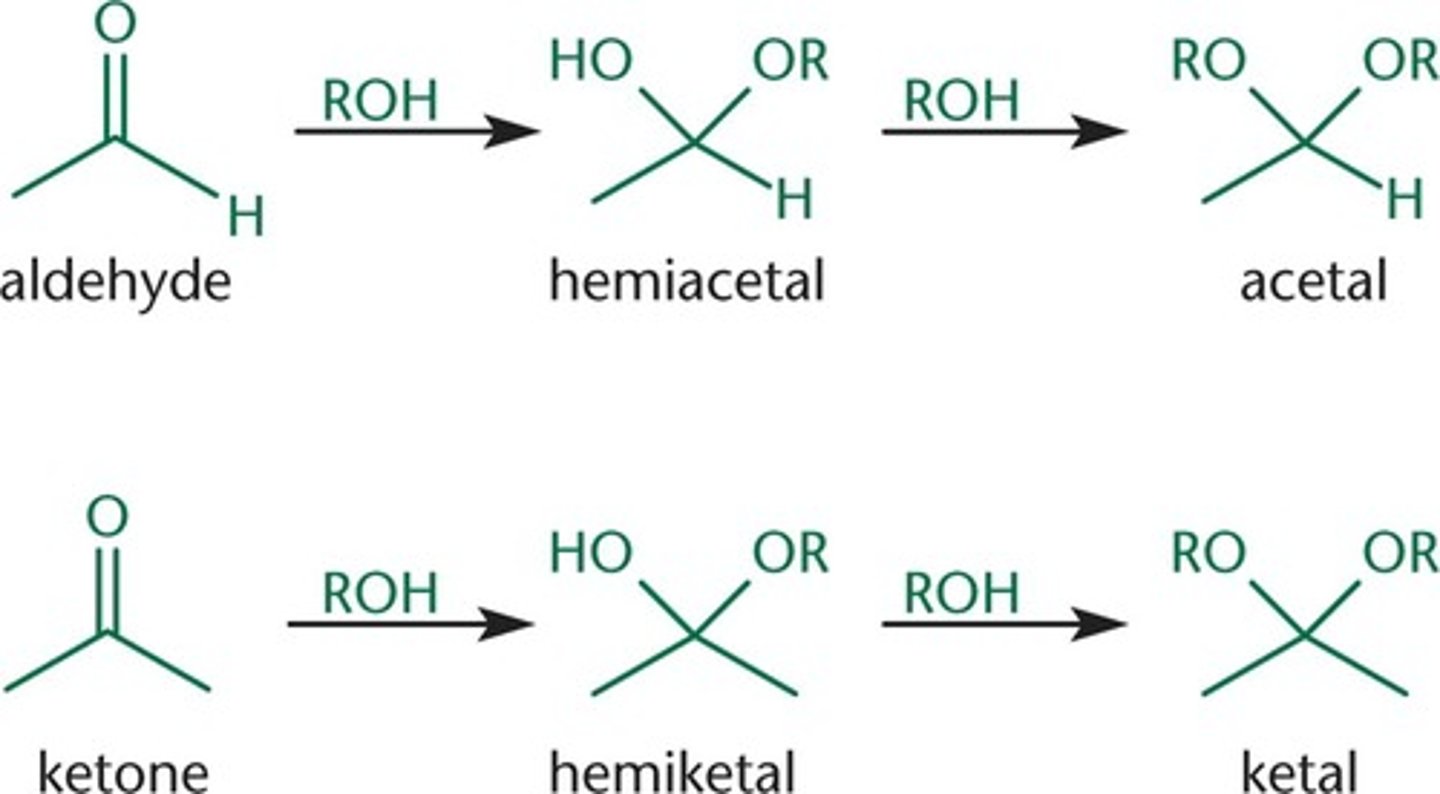
If a reaction occurs between a single OH group and a carbonyl group, a _______________ group will be generated. If a reaction occurs between two OH groups and a carbonyl group, a _______________ group will be generated.
(A) acetal/ketal, acetal/ketal
(B) acetal/ketal, hemiacetal/hemiketal
(C) hemiacetal/hemiketal, hemiacetal/hemiketal
(D) hemiacetal/hemiketal, acetal/ketal
(D) hemiacetal/hemiketal, acetal/ketal
If a reaction occurs between a single OH group and a carbonyl group, a hemiacetal/hemiketal group will be generated. If a reaction occurs between two OH groups and a carbonyl group, a acetal/ketal group will be generated.
Draw/visualize an acetal versus a hemiacetal. Also, draw or visualize a ketal versus a hemketal.
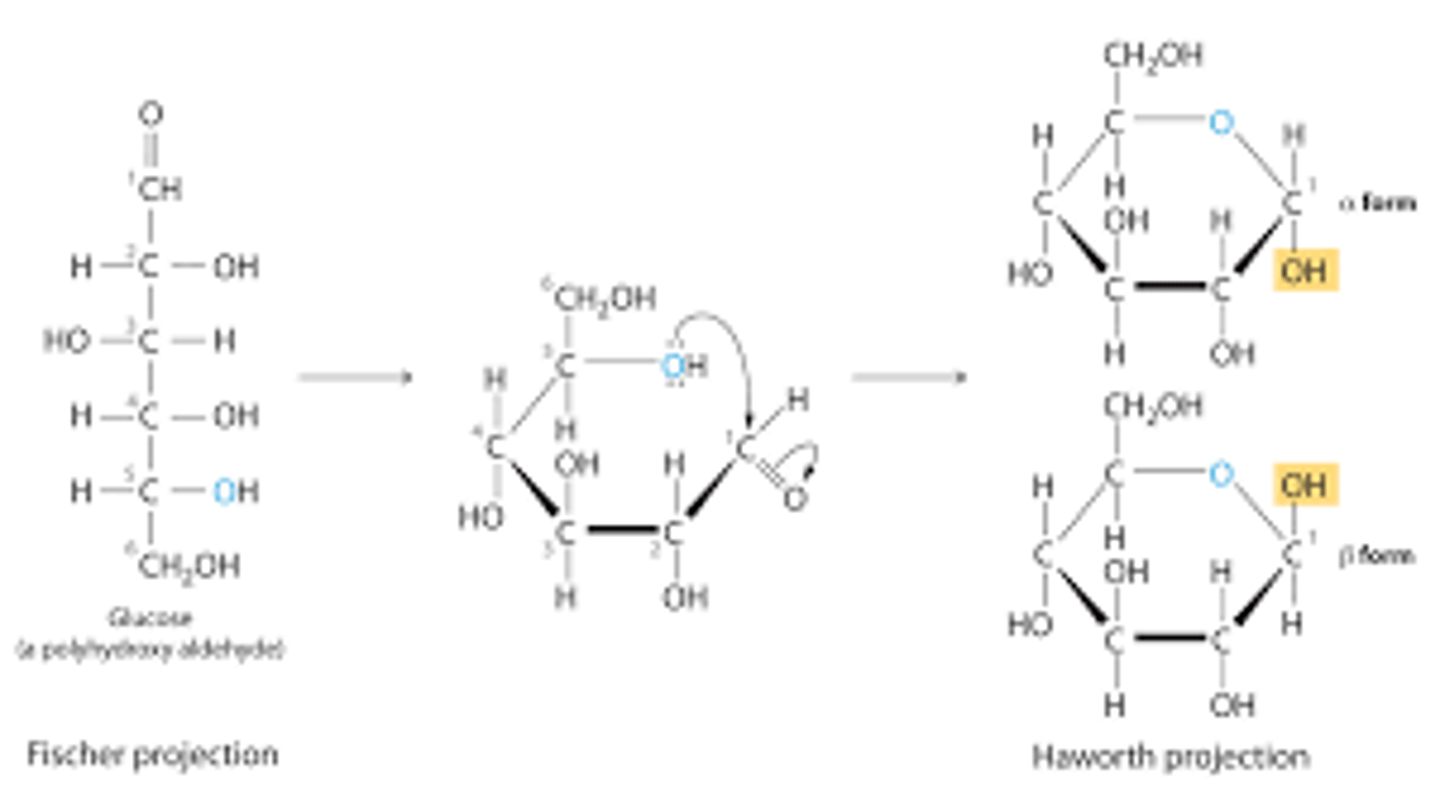
Draw or visualize D-glucose as a Fischer projection versus a Haworth diagram. Indicate which OH group attacks/attacked the carbonyl carbon in each.
The phrase "down right, up lefting" (which sounds like "downright uplifting") can help you remember that the groups on the right in the Fischer projection should point down in the Haworth diagram, and the groups on the left should point up.
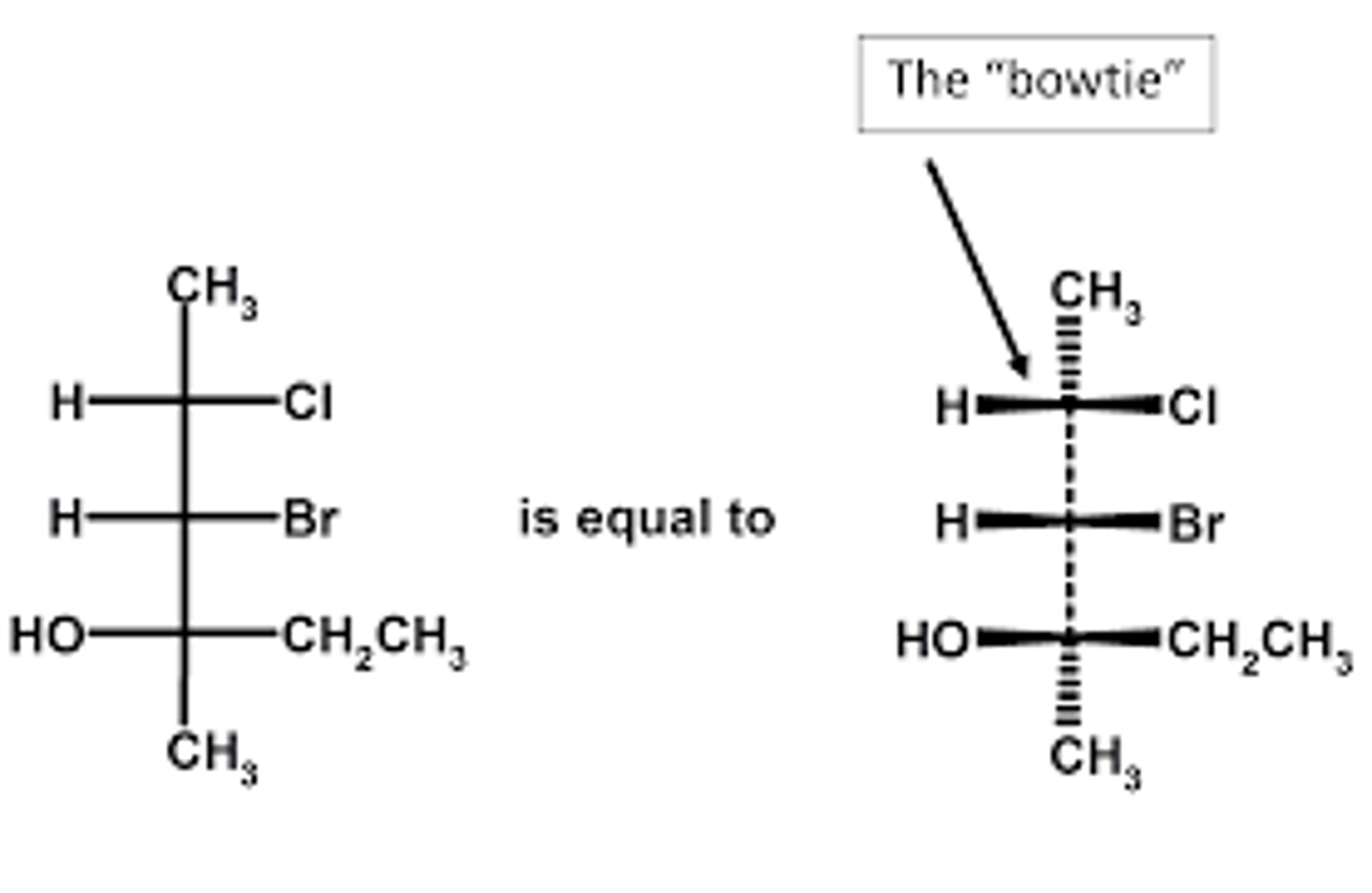
CRB Fill in the blanks: If you were trying to represent the Fischer Diagram with wedges and dashes, the __________________ would have dashes and the ______________ would have wedges.
(A) Left and Top, Right and Bottom
(B) Left and Right, Bottom and Top
(C) Top and Right, Left and Bottom
(D) Top and Bottom, Right and Left
(D) Top and Bottom, Right and Left
If you were trying to represent the Fischer Diagram with wedges and dashes, the Top and Bottom would have dashes (indicating that they are going into the page) and the Left and Right would have wedges (indicating that they are coming out of the page).
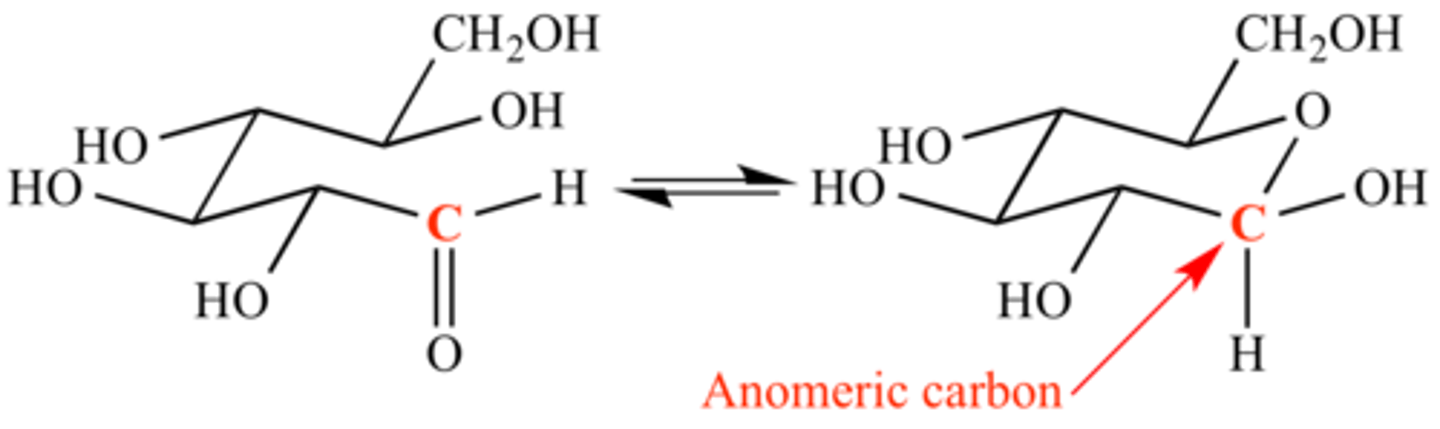
The carbon that was attacked by the OH group is known as the ____________ carbon.
(A) allosteric
(B) epimeric
(C) anomeric
(D) episteric
(C) anomeric
The carbon that was attacked by the OH group is known as the anomeric carbon.
Draw or visualize a pyranose and a furanose.
The key difference is that a pyranose is a 6-membered ring with one O in the ring, whereas the furanose is a 5-membered ring with one O in the ring.
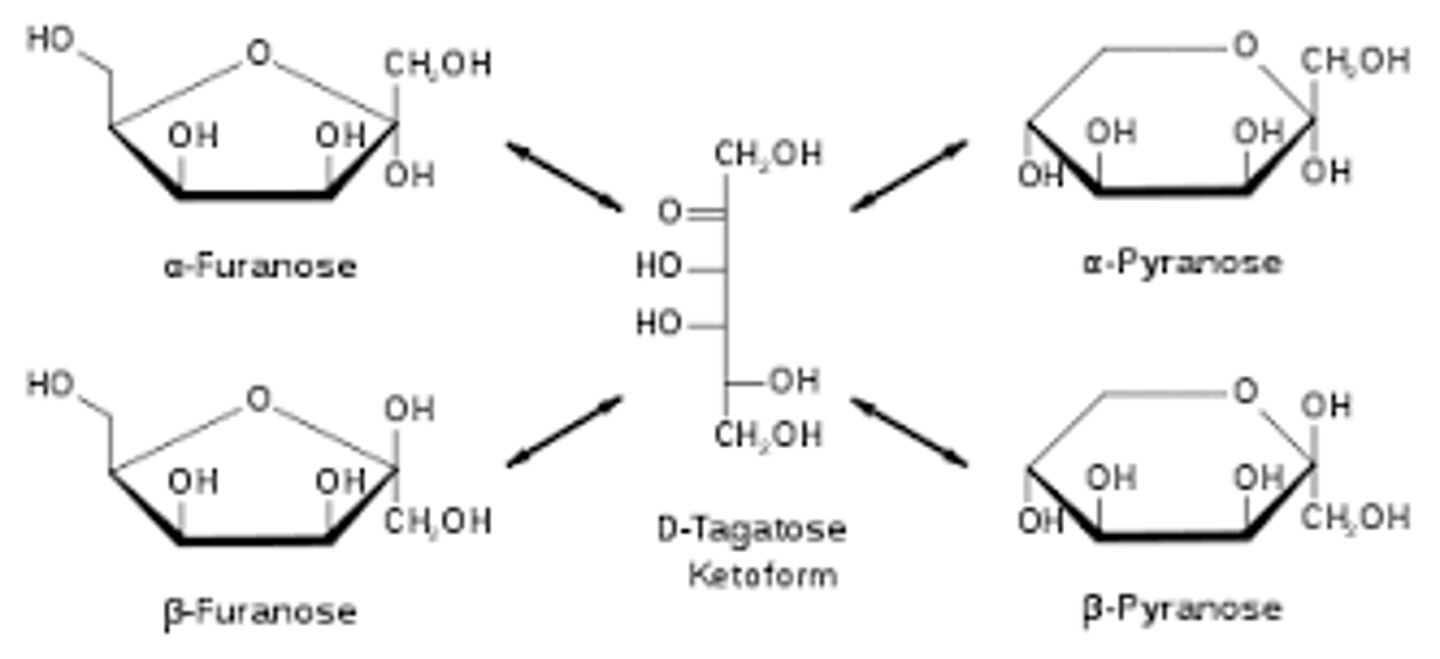
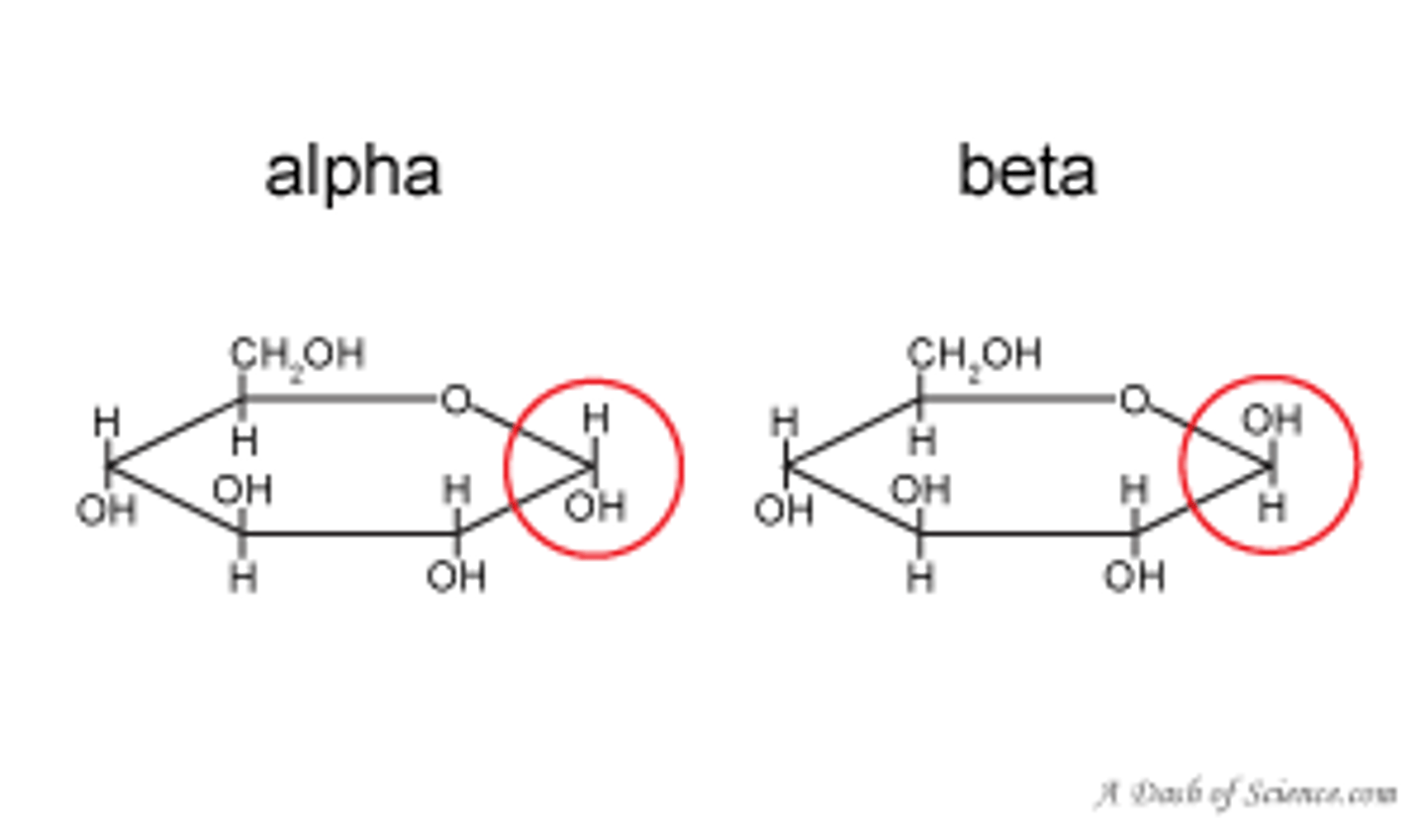
Draw or visualize D-glucose α and β anomers as Haworth projections. What differentiates the α from the β anomer?
In the α form, the OH group at the anomeric carbon points in the same (think "sαme") direction (up/down) as the OH on C2, and the opposite direction of the CH2OH flag group.
In the β form, the OH group at the anomeric carbon points in the same direction as the CH2OH flag group, and the opposite direction of the C2 hydroxyl.
Describe the process of mutarotation.
Mutarotation is when the ring-form of the sugar opens up and then recloses again. This may cause the sugar to bounce back and forth between the α and β anomers.
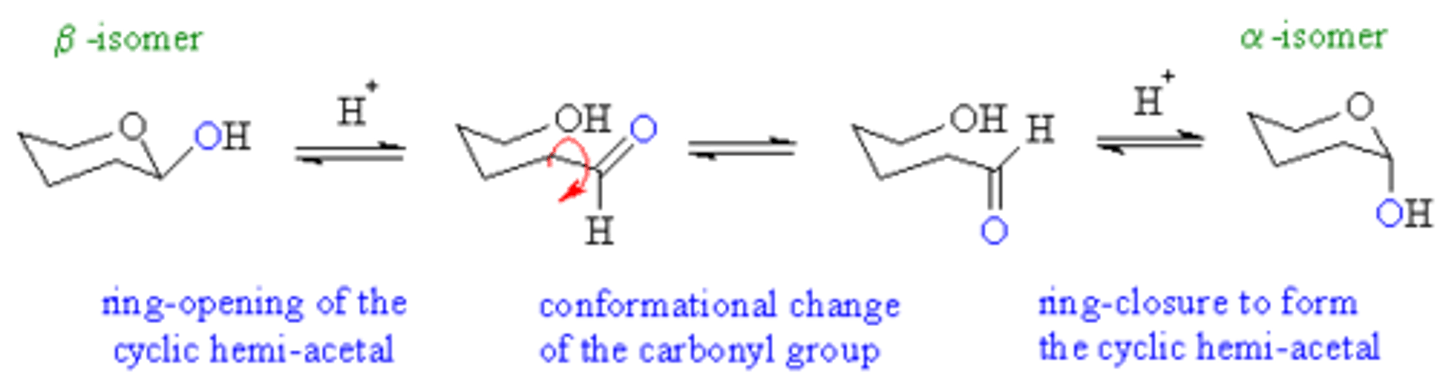
What does the naming convention for dissacharides of α-Man1,3-Gal indicate?
α indicates that the anomeric carbon (C-1) of mannose involved in this bond is an α anomer. 1,3 indicates that the bond is between C-1 of mannose and C-3 of galactose.
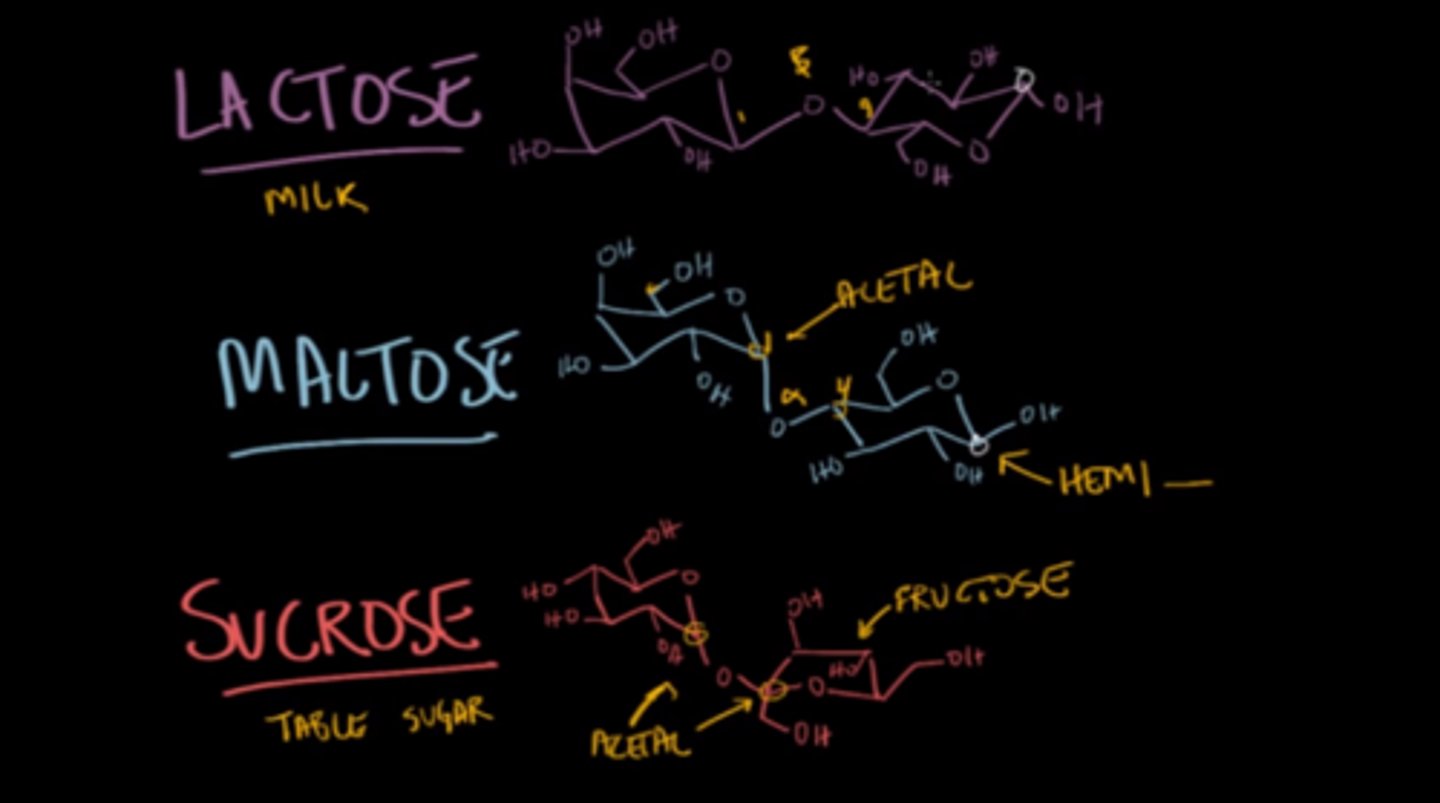
Lactose is a very important disaccharide found in milk. Which of the following best characterizes the structure of lactose? (draw it for extra credit)
(A) α-Glc1,4-Glc
(B) β-Glc1,4-Gal
(C) α-Gal1,4-Gal
(D) β-Gal1,4-Glc
(D) β-Gal1,4-Glc
β-Gal1,4-Glc is the structure of lactose.
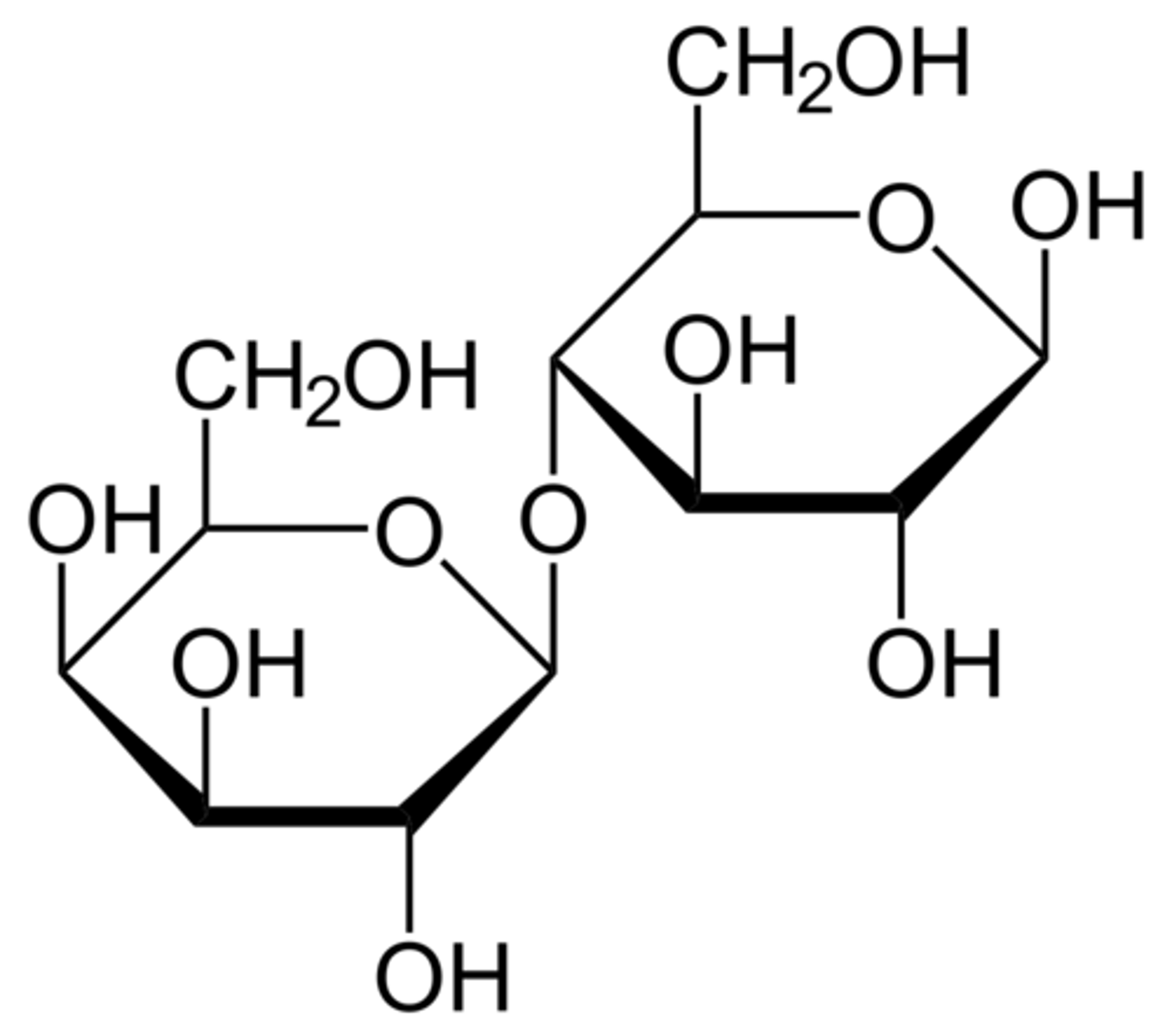
Maltose is a very important disaccharide. Which of the following best characterizes the structure of maltose? (draw it for extra credit)
(A) α-Glc1,4-Glc
(B) β-Glc1,4-Gal
(C) α-Gal1,4-Gal
(D) β-Gal1,4-Glc
(A) α-Glc1,4-Glc
α-Glc1,4-Glc is the structure of maltose.
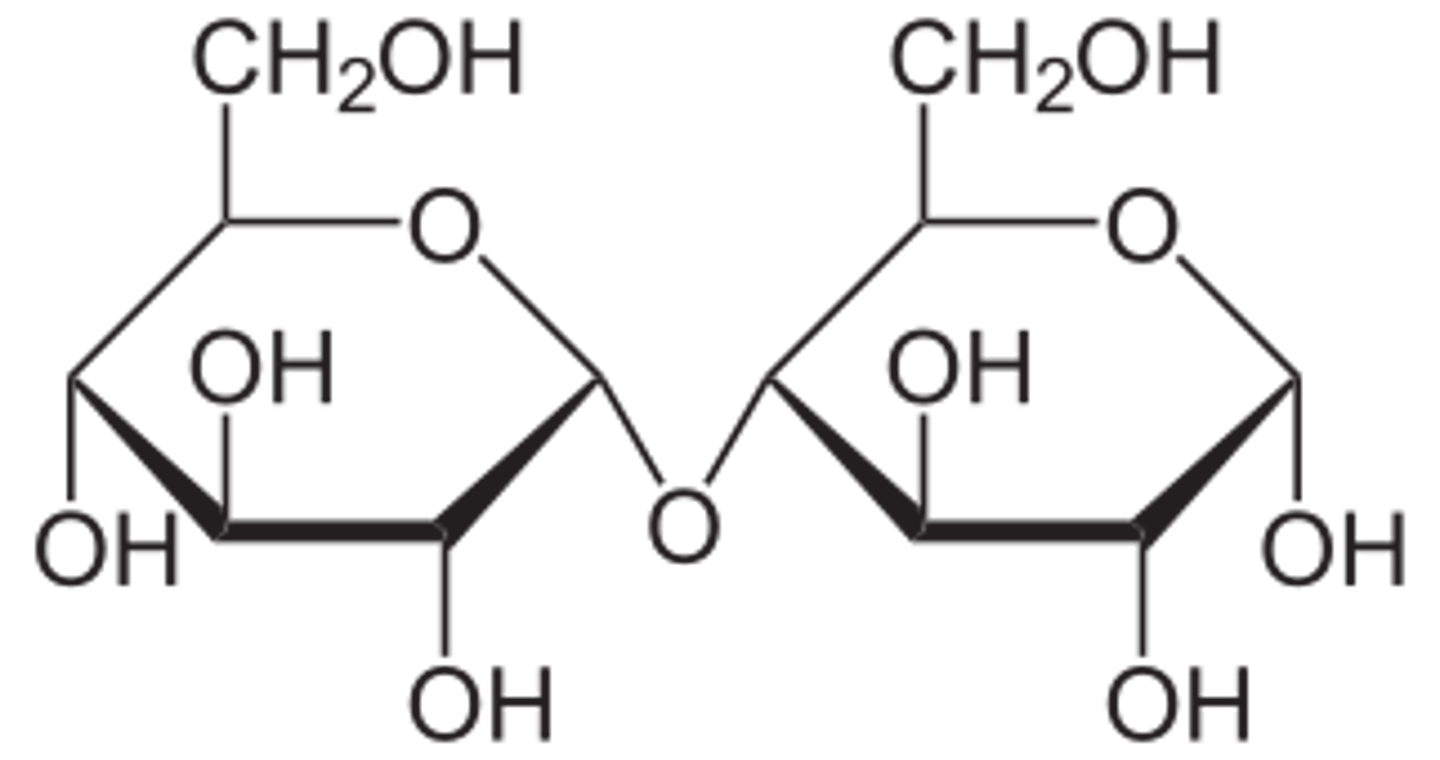
Sucrose is a very important disaccharide that is often found in table sugar. Which of the following best characterizes the structure of sucrose? (draw it for extra credit)
(A) β-Glc1,2-β-Fru
(B) α-Glc1,4-β-Fru
(C) α-Glc1,2-β-Fru
(D) β-Glc1,4-α-Fru
(C) α-Glc1,2-β-Fru
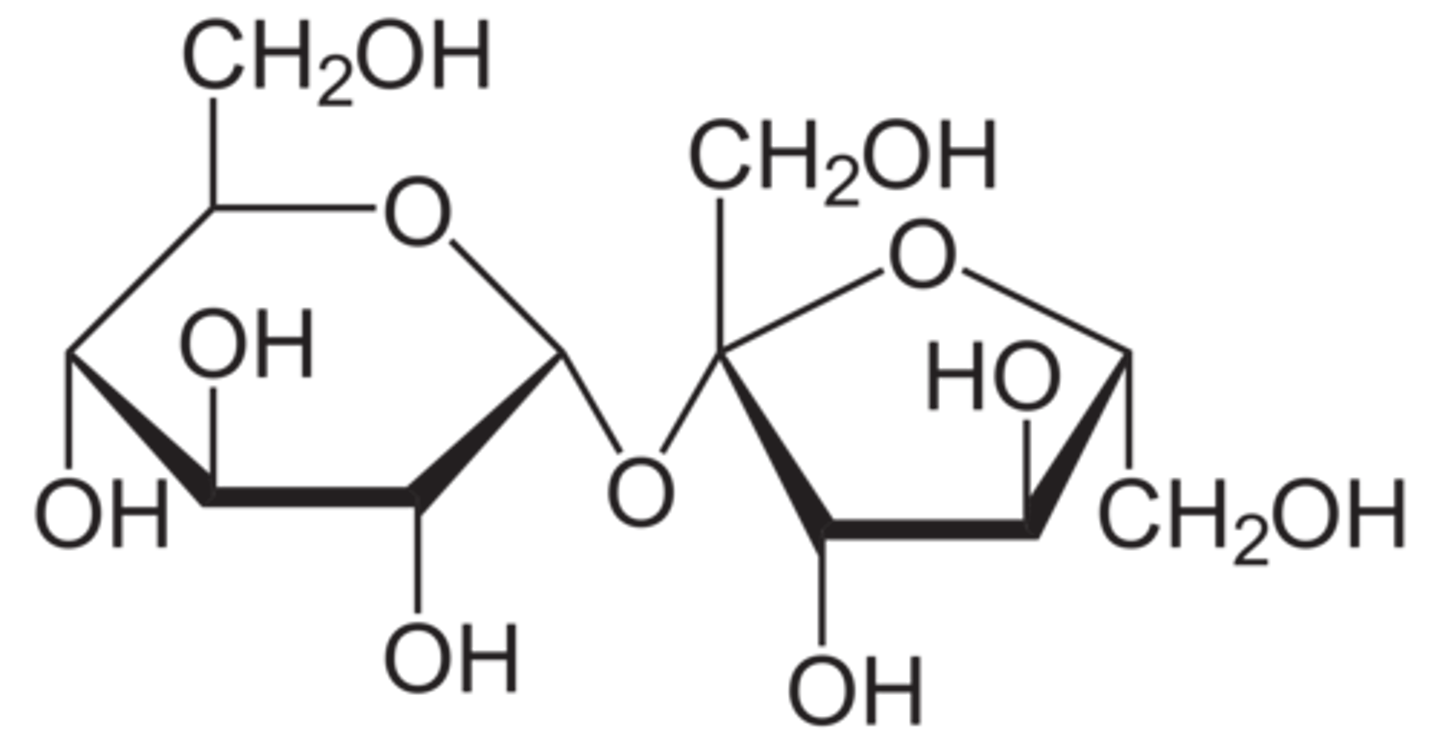
Sucrose has two acetal groups while lactose and maltose have one acetal group and a hemiacetal. Why is this the case?
This is due to the fact that in sucrose, the anomeric carbons of both sugar monomers are involved in the bond whereas in lactose and maltose the anomeric carbon of only one sugar monomer is involved.
Sucrose is considered a nonreducing sugar while lactose and mannose are considered reducing sugars. Why is this the case?
Lactose and mannose have an anomeric carbon that can undergo mutarotation to form an aldehyde group, which can be oxidized and act as a reducing agent.
Sucrose is considered a nonreducing sugar because it does not have an anomeric carbon available for mutarotation. Both sugar monomers are trapped in their cyclic forms.
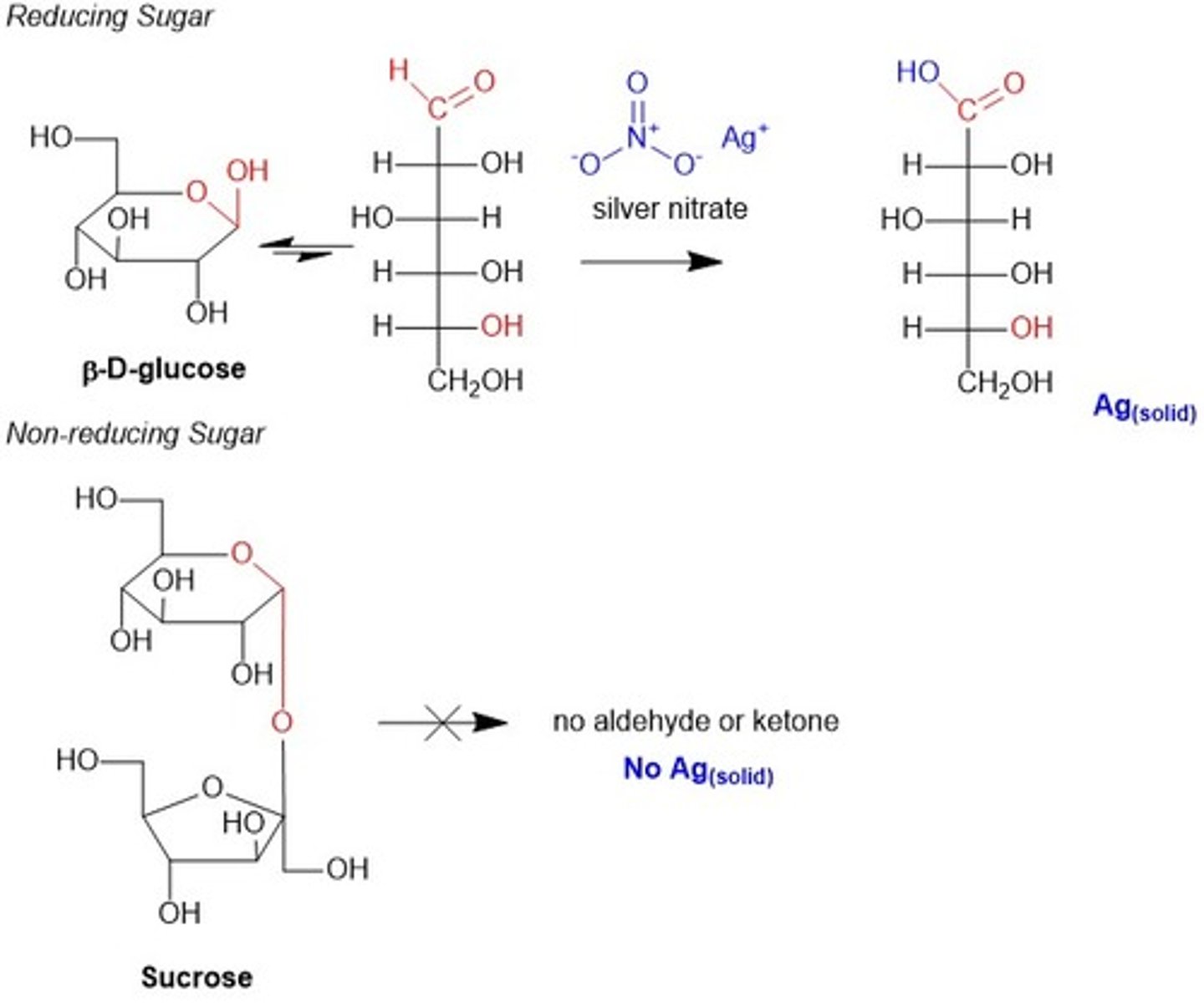
CRB True or false? Any monosaccharide with an Acetal Ring is considered a Reducing Sugar.
False. Any monosaccharide with a Hemiacetal Ring is considered a Reducing Sugar.
CRB Which of the following reagents are commonly used to test for reducing sugars?
I. Lindlar's
II. Tollen's
III. Benedict's
(A) I only
(B) I and III only
(C) II and III only
(D) I, II and III
(C) II and III only
Tollen's Reagent (Ag2O) and Benedict's Reagent (Cu2O) are both commonly used to test for reducing sugars.
Lindlar's Catalyst is often used to reduce Alkynes to Alkenes, and not all the way to Alkanes.
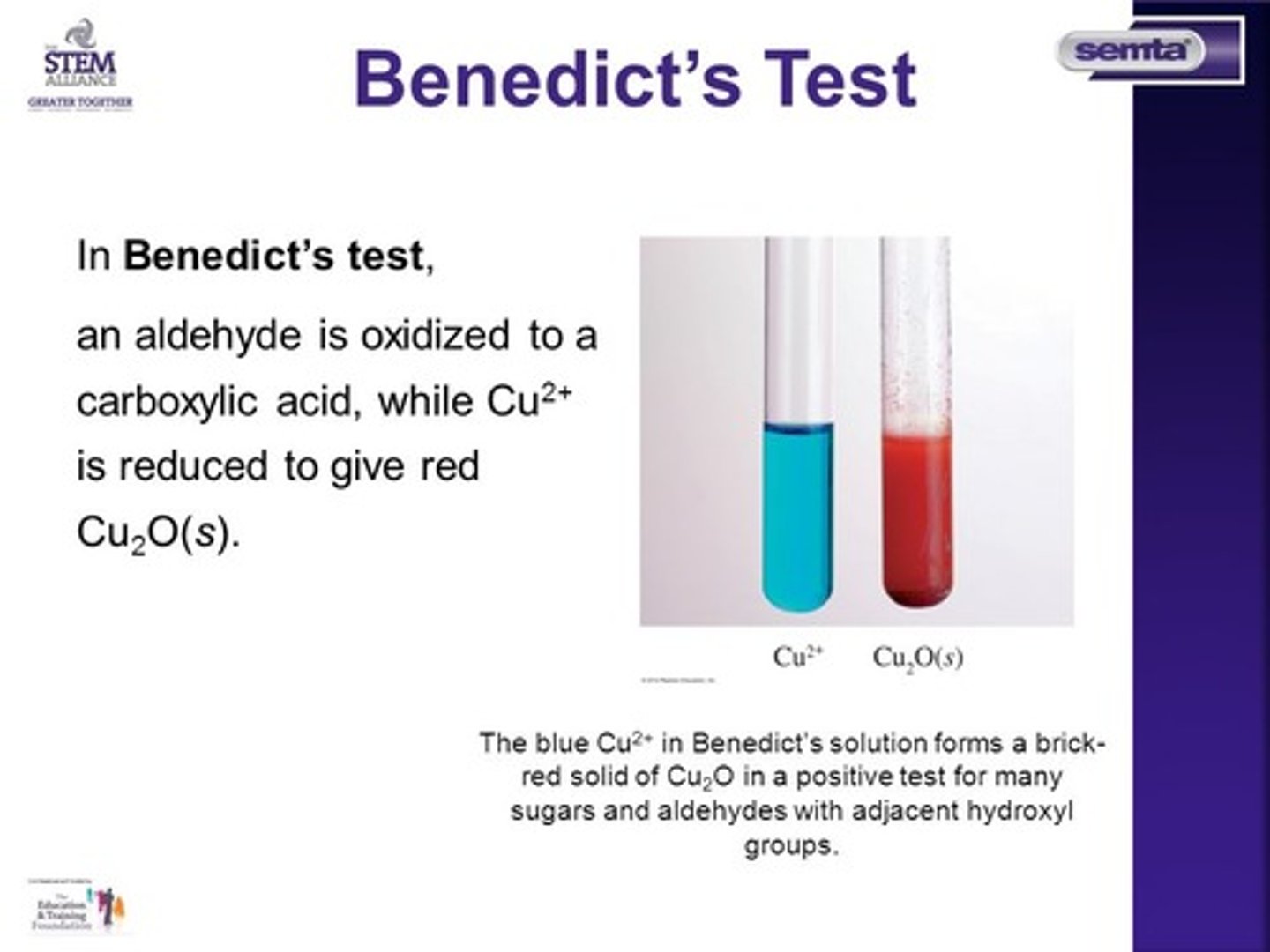
CRB Why can a more powerful oxidizing agent NOT be used to test for reducing sugars?
A more powerful oxidizing agent would be able to oxidize both the aldehyde formed during mutaroatation AND the primary alcohols. If it reacts with primary alcohols, even non-reducing sugars would react!
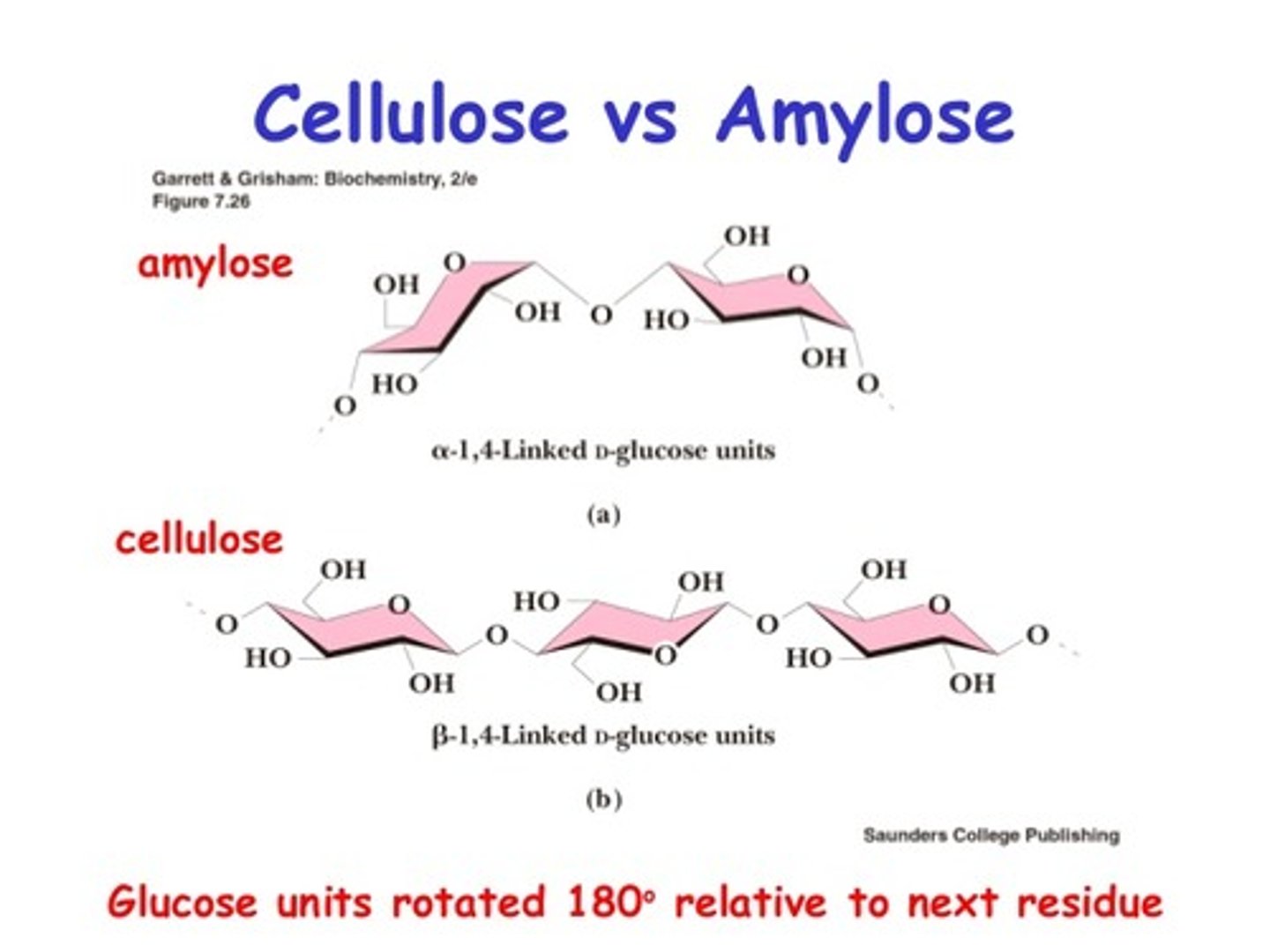
Compare the structure of cellulose and amylose (starch).
Cellulose is a polysaccharide composed of glucose with β-1,4 linkages.
Amylose is a polysaccharide composed of glucose with α-1,4 linkages.
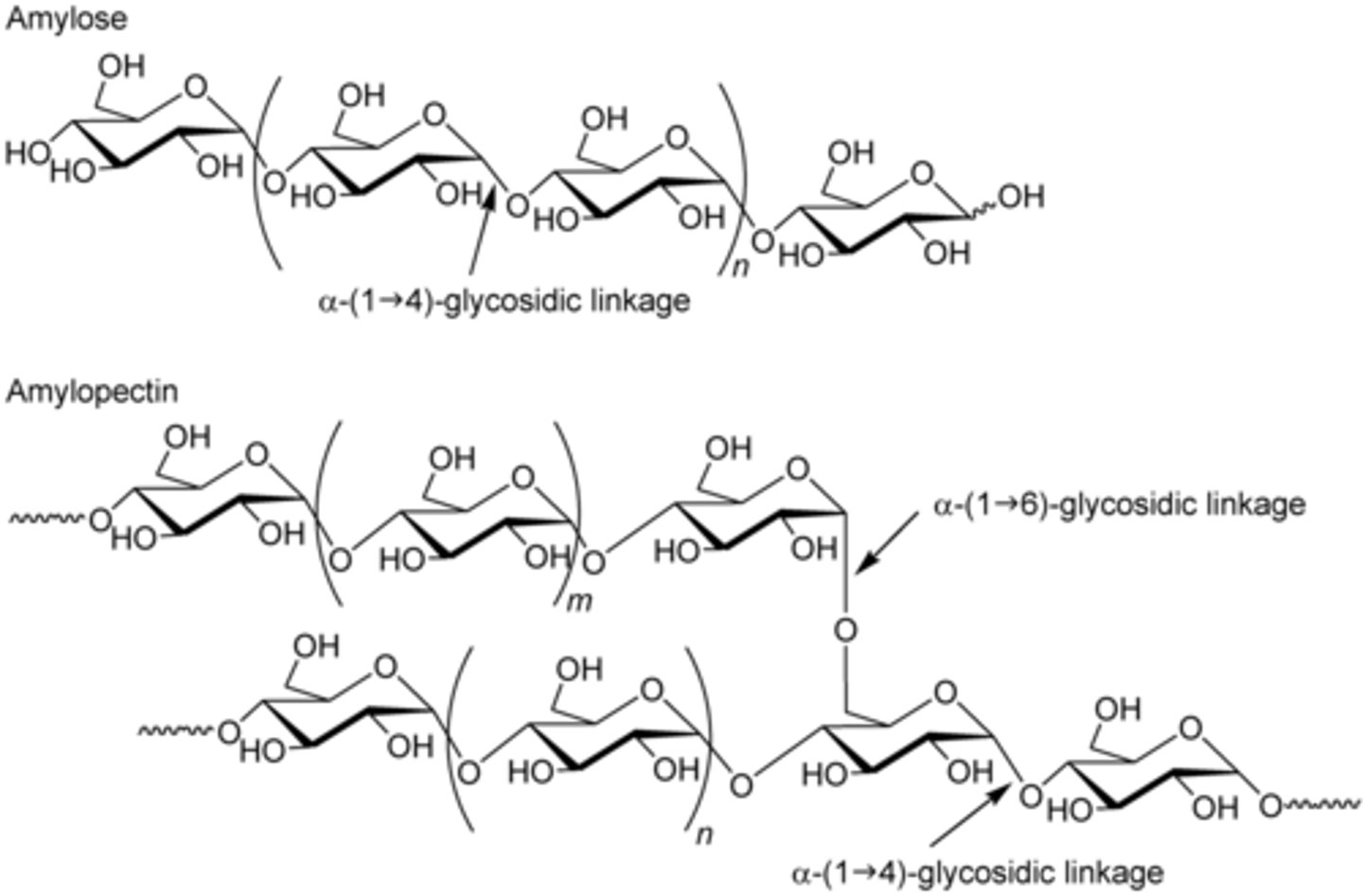
CRB Compare Amylose and Amylopectin.
Amylose is a linear polysaccharide linked by α-1,4 linkages.
Amylopectin is another polysaccharide, but along with the α-1,4 linkages, it also has branches with α-1,6 linkages.
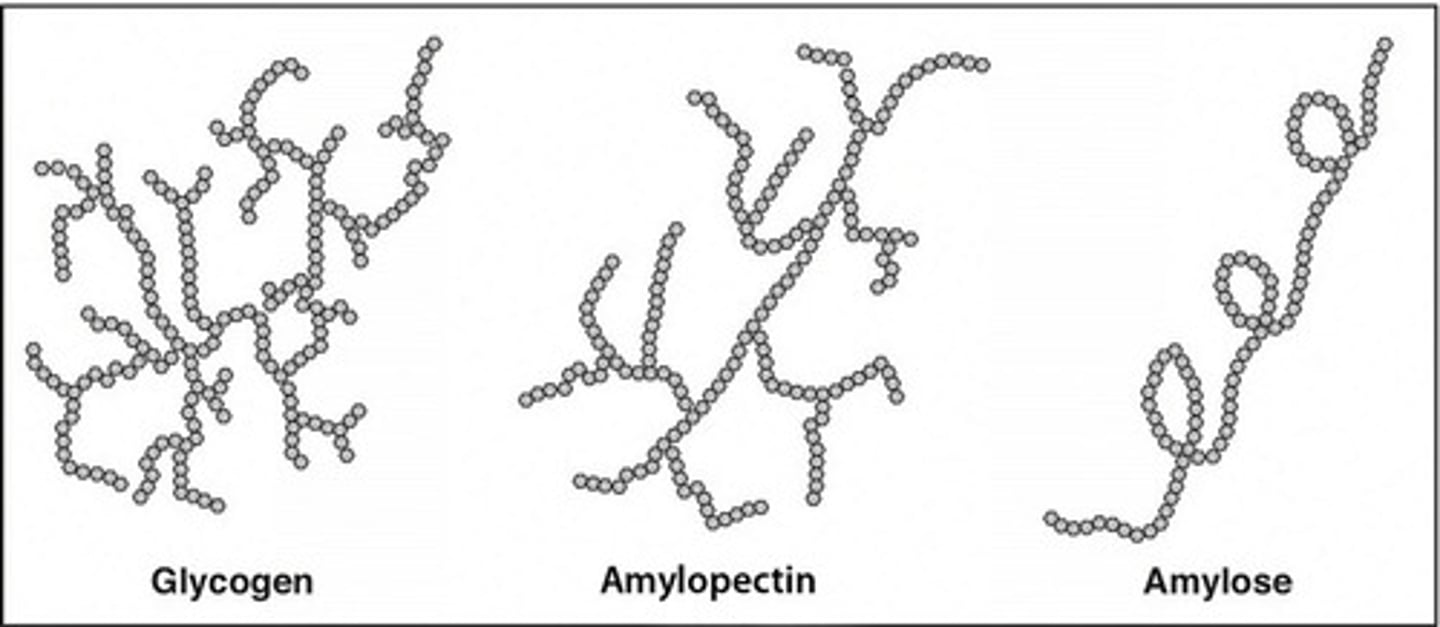
Why can humans break down starches (like glycogen and amylose) and their α-1,4 glycosidic linkages for energy but cannot break down cellulose and its β-1,4 linkages?
Humans lack the enzyme necessary to cleave a β-1,4 linkage, so they cannot break down cellulose for energy! We do have enzymes to cleave α 1,4 linkages, which is why we can digest them and utilize them as energy.
Compare the structure of amylose and glycogen.
Amylose is a polysaccharide composed of glucose with α-1,4 linkages.
Glycogen is a polysaccharide composed of glucose with α-1,4 AND α-1,6 linkages. This is the storage form of glucose for our cells.
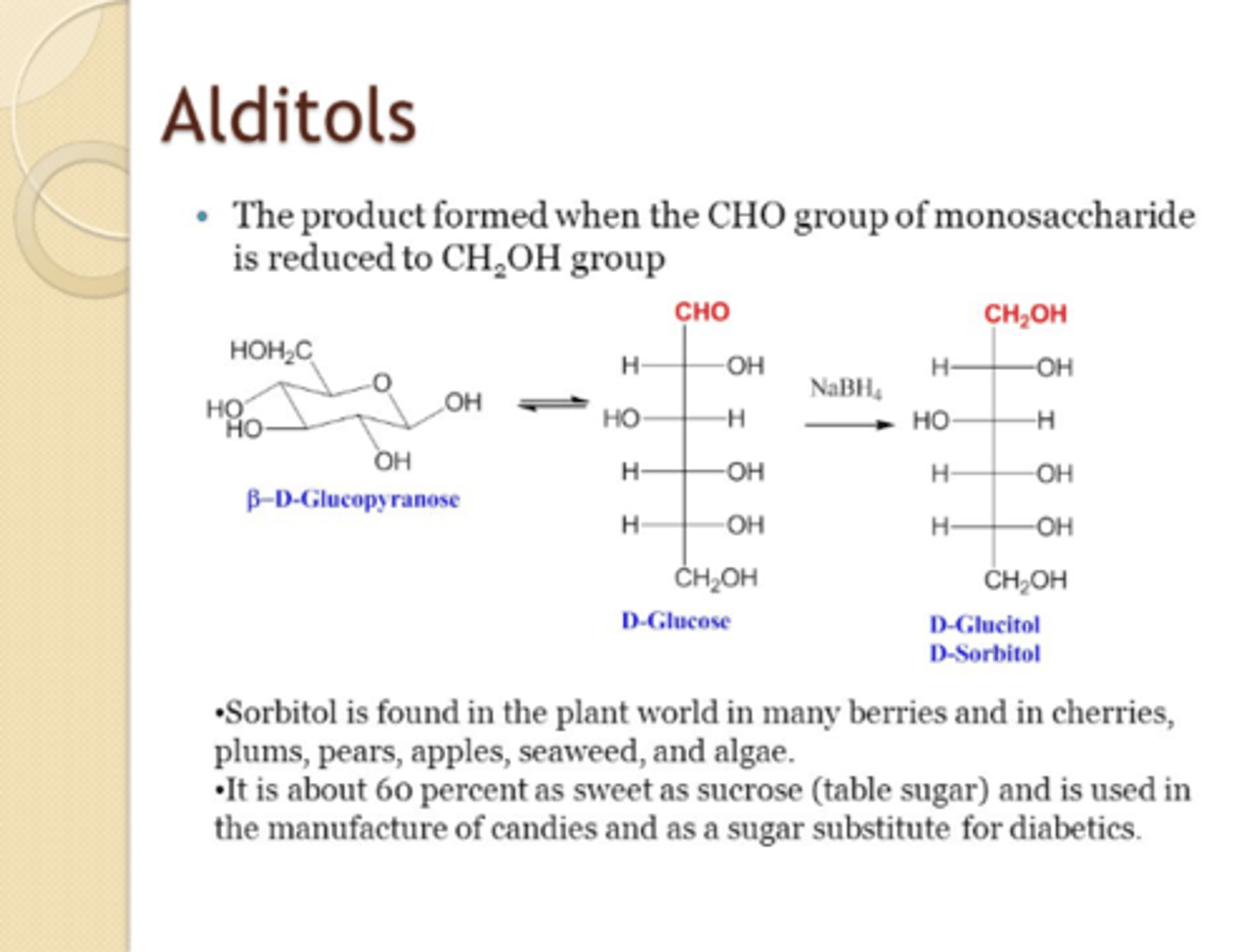
CRB Compare Alditols and Deoxy Sugars.
Alditols are the product of an aldose's carbonyl being reduced to an alcohol.
Deoxy Sugars are when a hydroxyl group is replaced by only a hydrogen, like in Deoxyribose.
CRB There are many people who cannot properly process lactose. Compare Lactose Malabsorbers and Lactose Intolerant people.
Lactose Malabsorbers are people who lack Lactase, meaning lactose will end up in the large intestine unprocessed.
Lactose Intolerant people will also end up with lactose in their large intestine, but they suffer from additional gas and soft stool due to having bacteria that will partially metabolize that lactose.