Respiratory Pharmacology and the Lungs as a Route of Drug Delivery
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
28 Terms
What two factors determine how a drug will distribute within the body?
Solubility determines how easily a drug dissolves and can move in body fluids.
Lipid-soluble drugs cross cell membranes easily and tend to accumulate in fatty tissues and inside cells.
Water-soluble drugs stay mainly in blood and extracellular fluid.
Ionization status (charged vs uncharged) determines membrane permeability.
Non-ionized (uncharged) drugs cross membranes readily and distribute into tissues and cells.
Ionized (charged) drugs cross poorly and are trapped in aqueous compartments (blood, urine, GI lumen).
Describe key anatomical features of the lungs and how this relates to their use a drug delivery route.
• Vast surface area, well perfused, so drugs can move into drugs or contact vast SA inside
• Can be used for:
systemic administration
e.g. general anaesthetic agents
targeted "local" therapy
e.g. bronchodilators, corticosteroids
Describe the features of a drug you’d expect to find with the following designated drug targets.
Local
Systemic
GA
• Local
• Want to retain drug at site of administration
• Charged molecule/limited lipid solubility
• Systemic
• Want drug to be absorbed into bloodstream
• Uncharged molecule/high lipid solubility
• General Anaesthetics need to reach the CNS since they induce their effect in the brain
• Need to get drug to site of action quickly to get to a stable state of anesthesia
• IV, inhalation - other routes too slow
• Lipid solubility key characteristic
What are some specialized drug delivery systems to enable drugs to reach the lungs?
Masks
Inhalers
Horses - obligate nasal breathers, therefore can simply cover the nostrils
How is anesthesia typically achieved (from a drug standpoint).
Anaesthesia is typically achieved using a combination of different drugs
Describe the combination of drugs used to achieve anesthesia.
1. PREMEDICANT DRUGS
• Drugs given prior to a general anaesthetic
• Typically a sedative-opioid combination
2. INDUCTION DRUGS
• Usually intravenous agents
• Used to achieve the transition from consciousness to unconsciousness
3. MAINTENANCE DRUGS
• Usually inhalational agents
• Drugs used to maintain the anaesthetic state
What is the Overton and Meyer Lipid Theory?
Proposed by Hans Meyer and Charles Overton, it states that the potency of many anesthetic drugs is directly proportional to their lipid solubility. In simple terms, suggested that General Anesthetics worked by: the more lipid-soluble a drug was, the more easily it produces anesthesia, because it partitions into cell membranes.
When lipid-soluble drugs insert into membranes, they can:
Increase lipid fluidity
Alter bilayer thickness and dimensions
Increase membrane permeability
These physical changes were thought to disrupt normal membrane function, especially in neurons, thereby reducing excitability and causing anesthesia.
What are the arguments for and against the Overton and Meyer Theory?
Arguments for:
Correlation:
Anesthetic potency correlates strongly with lipid solubility across many agents, supporting the idea that action occurs in membrane lipids.Pressure reversal:
High pressure reverses anesthesia, consistent with the idea that anesthesia results from expanded or more fluid lipid membranes, which pressure can compress back to normal.
Arguments against:
Temperature:
Increasing temperature also increases membrane fluidity, yet it does not mimic anesthesia, arguing against fluidity alone causing anesthetic effects.Stereoselectivity:
Optical isomers with identical lipid solubility can have different anesthetic potencies, implying action at specific protein targets, not just lipids.Cut-off phenomenon:
In homologous series (e.g., alcohols), anesthetic potency increases with lipid solubility only up to a point (cut off), after which larger molecules lose activity as a general anesthetic despite high lipid solubility.Correlation with enzyme inhibition:
Anesthetic potency correlates better with protein/enzyme inhibition than with lipid effects, suggesting direct interaction with proteins (ion channels, receptors).
What is the current view of lipid solubility of both inhalational and IV agents?
Modern understanding sees anesthetics working through defined receptors/protein targets, with lipid effects playing a secondary, modulatory role
Lipid solubility still important in reaching target
What is the Blood:Gas partition coefficient?
What does a low b:g value tell you?
What does a high b:g value tell you?
Blood:gas partition coefficient (b:g)
The blood:gas partition coefficient is the ratio of anesthetic concentration in blood to that in alveolar gas at equilibrium.
It reflects how soluble the anesthetic is in blood, which determines the speed of induction and recovery:
Low b:g:
Anesthetic is poorly soluble in blood.
Blood saturates quickly because it can’t “soak up” much drug.
Alveolar partial pressure rises rapidly, and the brain partial pressure follows, leading to fast induction and recovery.
High b:g:
Anesthetic is highly soluble in blood.
Blood acts like a large sponge, taking up a lot of anesthetic without much rise in partial pressure.
Alveolar and brain partial pressures rise slowly, leading to slow induction and recovery.
Key point:
Blood:gas coefficient determines speed of anesthesia: low = fast, high = slow.
What is the oil:gas partition coefficient?
What does a high o:g value tell you?
What does a low o:g value tell you?
Oil:gas partition coefficient (o:g)
The oil:gas coefficient is the ratio of anesthetic concentration in lipid (oil) to gas at equilibrium.
It reflects lipid solubility and potency:
High o:g:
Drug is highly lipid-soluble → high potency → lower MAC required.
Low o:g:
Drug is less lipid-soluble → lower potency → higher MAC required.
Key point:
Oil:gas coefficient determines potency, not speed.
What changes the speed of induction / recovery considering the drug concentrations?
Speed of induction and recovery depends on how fast anesthetic concentrations rise and fall in the brain after reaching lungs, which is influenced by:
Properties of the Anesthetic (Drug)
Blood:gas partition coefficient
Measures how soluble the anesthetic is in blood.
Low blood:gas → less uptake into blood → alveolar and brain partial pressures rise quickly → rapid induction and recovery.
High blood:gas → slower induction/recovery.
Oil:gas partition coefficient
Reflects lipid solubility and potency.
High oil:gas → more potent (lower concentration needed), but does not determine speed.
Physiological Factors
Alveolar ventilation
Increased ventilation delivers anesthetic to alveoli faster → faster induction.
How quickly is air breathed in and out?
Cardiac output
High cardiac output removes anesthetic from alveoli into blood more quickly → slower rise in alveolar/brain concentration → slower induction.
Low cardiac output → faster induction.
How are most inhalational agents metabolized?
• metabolized by liver - extent depends on agent
• metabolism has little influence on duration of action
Vast majority are breathed out, only a few are metabolized, therefore little influence here
• potential toxic metabolites risk to patient and staff produced by metabolism of some drugs
Significant in animal who is getting repeated anesthesia over a short period of time, which can build up
How are most inhalational agents eliminated?
• primarily by exhalation, rather than metabolism or other routes
• exhalation also determines duration of action, as once stopped being administered, patient clears drug = allows them to wake up
What is a vaporizer and which drugs is it designed to be used with?
A vaporizer is a device that turns liquid anesthetic into a controlled concentration of vapor and delivers it in the inhaled gas mixture.
Works specifically with volatile (halogenated) anesthetics, which are liquids at room temperature but vaporize easily.
What are the Halogenated Anesthetics?
• Halothane
• Isoflurane
• Sevoflurane
• Desflurane
What are the true gases used as general anesthetics?
• Nitrous oxide
• Xenon.
What is the MAC value?
Minimum Alveolar Concentration (MAC) is a standard measure of anesthetic potency, the dose.
Definition:
Is the concentration of an inhaled anesthetic in the lungs that prevents movement in 50% of people in response to a specific stimulus.
Low MAC = higher potency, less drug needed to achieve anaesthesia = stronger drug
MAC compares the potency of different inhalational anaesthetics
Relates to the percentage of drug in the inspired air
What are the factors that can alter MAC values?
• Species
• Age - MAC lower in 'geriatrics' & neonates (Drug more potent in these patients)
• Pregnancy - MAC reduced
• Hypothermia - MAC reduced
• Drugs - premedicants can greatly reduce MAC
As drugs can potentiate the GA
What do the following features have the ability to tell you about an inhaled anesthetic?
Vapour Pressure
High vapor pressure → drug vaporizes easily
Low vapor pressure → easier to control with standard vaporizers
MAC and Oil:gas = inverse
Blood:gas partition
Low b:g - easy to adjust depth
High b:g - harder to adjust depth quickly

Summarize main features of the SNS and PNS.
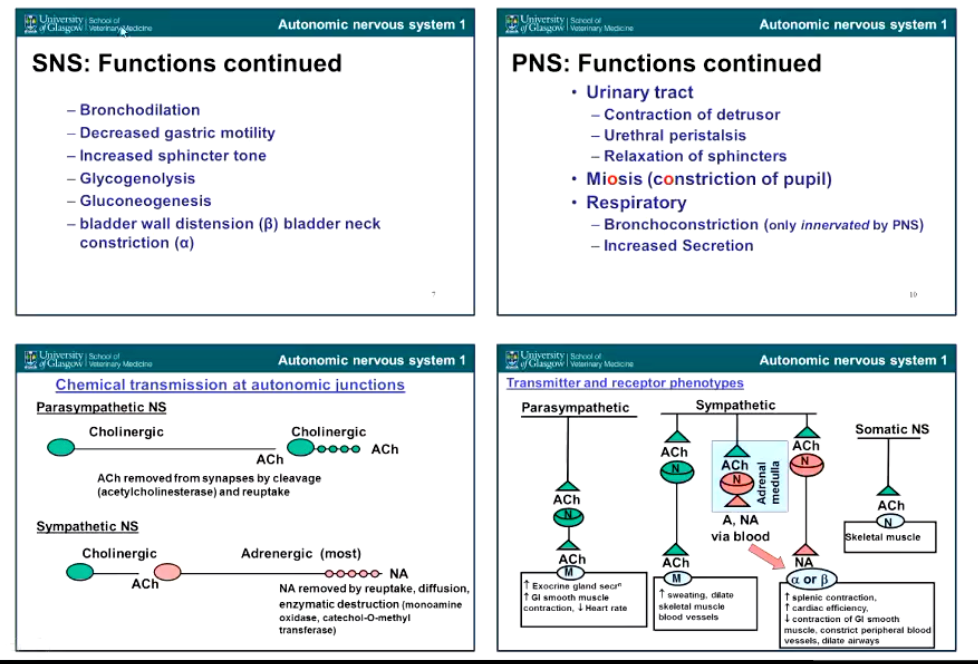
Describe the Sympathetic Control of bronchial tone.
• no direct innervation of the sympathetic nervous system into the lungs but lungs are dilated by circulating adrenaline, stimulates:
• B2 adrenoceptors - GPCRs causing
• increase cAMP in bronchial smooth muscle, activates protein Kinase A which has an inhibitory effect and causes
• relaxation of bronchial smooth muscle
Also inhibits release of histamine from mast cells (useful in allergic conditions)
Describe the parasympathetic Control of bronchial tone.
Vagal stimulation - bronchial constriction due to rest/digest state, requiring reduced oxygen intake
• Muscarinic acetylcholine receptors (Specifically M3 - GCPR)
Leads to increase IP3
increase [Ca2+]
Calcium causes constriction of bronchial smooth muscle
Summarize the smooth muscle contractile pathways:
Noting B2 and M3
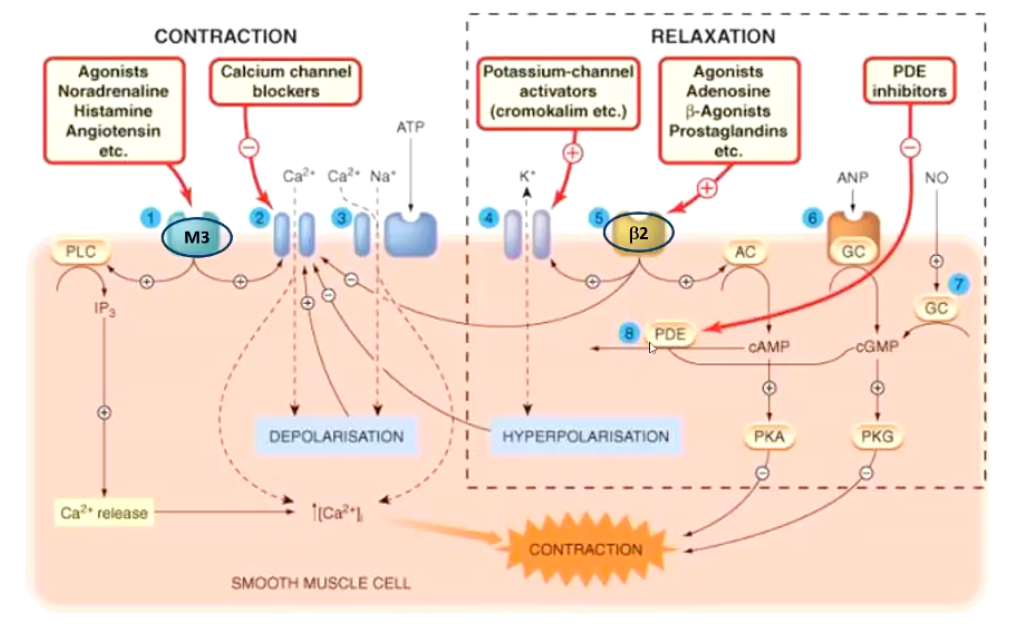
What are the ways in which we can achieve bronchodilation pharamacologically?
• Drug Targets
• B-adrenergic agonists
• Anticholinergic (antimuscarinic) Drugs
• Methylxanthines (PDE inhibitors)
PDE inhibitors prevent the breakdown of cAMP or cGMP → higher levels of these messengers → smooth muscle relaxation.
• Targeted drug delivery systems
Describe the main B-Adrenoreceptor Agonists, their main functions and side effects.
Adrenaline (epinephrine)
• Emergency treatment of life-threatening bronchoconstriction
Not for chronic use
B2 adrenoceptor specific agonists
e.g. terbutaline, salbutamol, salmeterol, clenbuterol
Mechanism: Stimulate adrenergic pathways
Some have fewer side effects, better for chronic conditions
overall relaxation of bronchial smooth muscle
Side effects
• CVS: increased heart rate, palpitations
• Skeletal muscle: tremors
Also see tolerance → causing a loss of efficacy over time
Describe the main Anticholinergic (antimuscarinic drugs), the mechanism, side effects and administration.
Mechanism: Block endogenous parasympathetic tone (Bronchoconstriction) by inhibiting muscarinic receptors (antimuscarinic), leads to Smooth muscle relaxation → bronchodilation
e.g. atropine
Side effects:
• CNS stimulation
• Gl inhibition
Can use targeted Administration: topical vs systemic
Local effect:
Drug called: Ipratropium bromide quaternary derivative (Always charged) of atropine, limited absorption therefore minimal systemic side effects
Describe the main Methylxanthines, the mechanism and side effects.
e.g. theobromine, caffeine, theophylline, etamiphylline
Mechanism of action
PDE inhibitors
→ increase CAMP by preventing it’s break down, allowing it to prevent contraction, leading to:
→ bronchial smooth muscle relaxation
• Also
decrease inflammatory mediators
adenosine inhibition → (Adenosine also causes constriction so further allows relaxation)
Side effects
• GI - nausea, vomiting
• Cardiac - increased rate + force of contraction
• CNS - alertness, agitation, nervousness