Biochem
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
80 Terms
The atom
made up of a nucleus that contains protons (positively charged subatomic particles) and neutrons (neutral particles), surrounded by electrons (negatively charged subatomic particles) that orbit the nucleus.
Atomic number
is the number of protons in the nucleus of an atom, which defines the element and its position on the periodic table.
→ # of electrons in orbital = protons in neutral atoms ( they have to be the same amount + equal a whole number)
Atomic mass
is equal to the number of protons & neutrons in the nucleus
Isotopes
are variants of a particular chemical element that have the same number of protons but different numbers of neutrons, resulting in different atomic masses.
Element
A substance made up of only one type of atom, all which have the same atomic number
Energy levels
The distances of electrons from the nucleus depending on its energy content ( the rings)
→as an electron moves from one energy level to a more distant level its potential energy increases
Orbital
The specific volume of space around an atomic nucleus where an electron is likely to be found.
→ each one can contain up to 2 electrons at a given time.
→A pair of electrons are more stable then 1
→An energy level can contain more then 1
→ 1st energy level has 1 (holds max 2 electrons), 2nd energy level has 4 (holds max 8 electrons)
Valence Shell
The outermost energy level, contain valence electrons. these electrons determine the chemical behaviour of the atom depending on how many there are. The fuller the valence orbital the more stable it is. Atoms without “full” electrons will try to lose or gain electrons in order to stabilize
→ losing an electron = positivly charged
→ gaining an electron=negatively charged
Ions
is a charged atom (negative or positive)
Compounds
substances composed of 2 or more elements in a set ratio. eg, water is composed of 2 hydrogen and 1 oxygen atom
organic compounds
compounds that contain carbon
Carbon element
facts
→ It’s atom contains 4 valence electrons in its orbitals
→can form 4 stable covalent bonds with these electrons. when it’s atom is joined with 4 other atoms (eg. 4 H atoms) it forms a tetrahedral shape
→ when they bond with other elements their molecules look straight, bent, or even ring shaped
Hydrocarbon
an organic compound that is only composed of carbon and hydrogen atoms (the longer ones tend to be non-polar due to symmetry in their bonds)
eg. pentane
Functional groups
different elements which can be attached to any of the carbons in an organic molecule
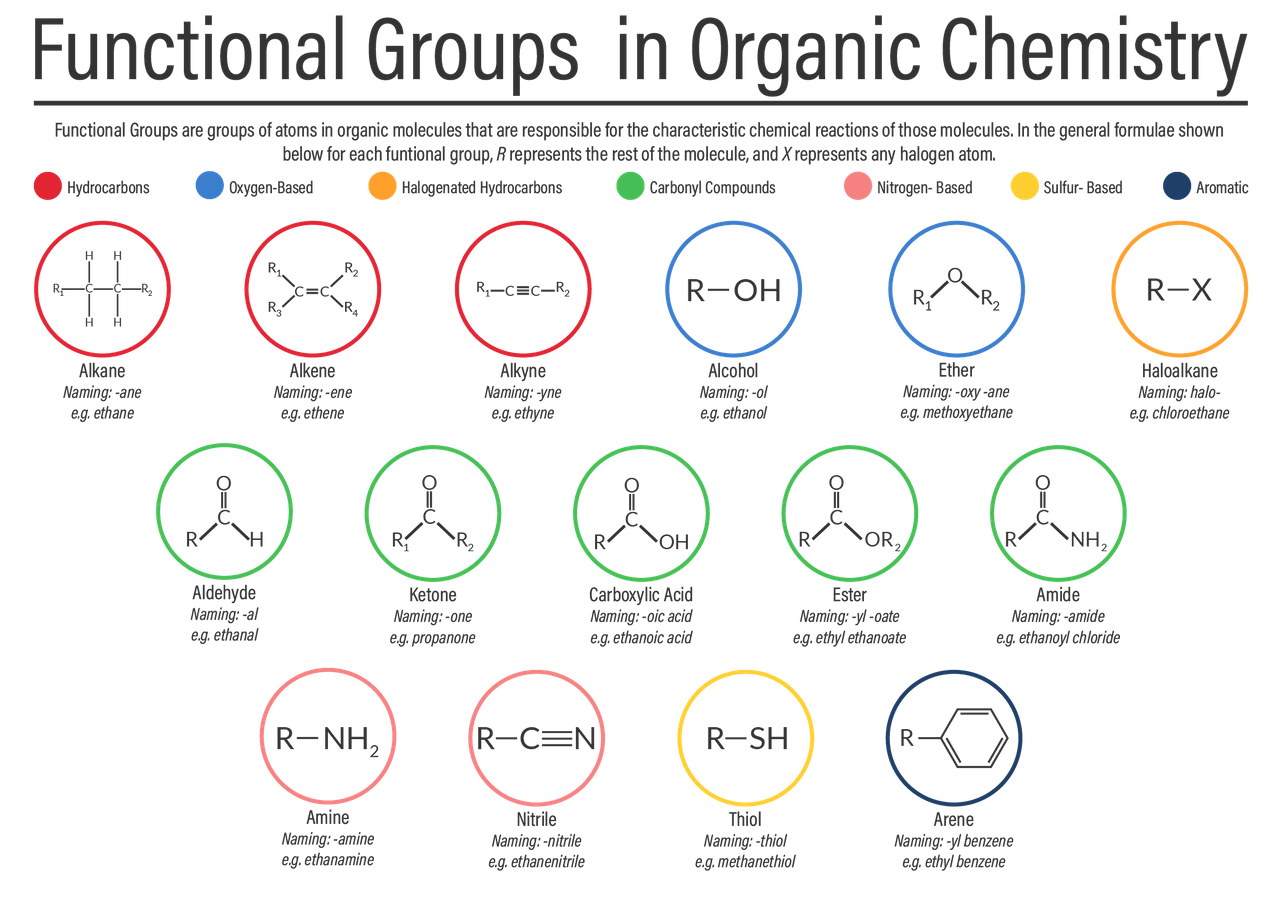
Monomers
are atoms or small molecules that bond together to form more complex structures such as polymers
four categories of carbon polymers (in living organisms)
→ Nucleotides
→proteins
→fats
→carbohydrates
Name some important carbohydrate facts
→#1 food and energy source for living organisms
→General formula: Cn(H2O)n, ex: C6H12O6
→sugars are the most simplest form, and are the major storage molecule for living organisms
Starch
A long polymer of glucose molecules aligned in the same direction. It’s how plants store energy & a major energy source for humans
Amylose & Amylopectin similarities
The 2 forms of starch found in our food
They both are polysaccharides containing multiple glucose units that are connected by glycosidic linkages
amylose
→ forms a straight chain
→ when a hydroxyl group on carbon 1 of one glucose molecule reacts within hydroxyl group on carbon 4 of a neighbouring glucose molecule to form water and a glycosidic linkage
Amylopectin
→made up of many short straight chains
→some straight glucose chains forms branches when the hydroxyl groups on carbon 6 and carbon 1 of different chains react, creating a glycosidic bond and releasing water
Glycogen
A type of glucose polymer
→is the long term food storage molucule in animals
→its found in the liver +muscles of humans
Cellulose
A long polymer of glucose molecules, with every other glucose flipped upside down. This slight structural tweak makes it ideal for providing structure support in plants
→ can’t be digested by humans unlike starch and glucose
→its referred to as fibre, which helps in digestion by aiding material movement through the gut
Lipids
A large diverse group of naturally occurring organic compounds that are related by the fact that they are insoluble in water. They have great structure variety
fatty acids
are the simplest lipids, featuring a long hydrocarbon chain and a carboxyl group (-COOH) at one end.
→ their chains usually have 12-24 carbons, are linear, and typically contain an even number of carbons
saturated fatty acids
has no double bonds, so each carbon holds all possible hydrogen atoms (max capacity). They are usually solid at room temp
→ ex: palmitic and stearic acid
Unsaturated fatty acids
DOESN’T hold the maximum possible hydrogen atoms that they can.
→ contains 1 or more carbon-carbon double bonds, casually kinks in their chains
→these kinks prevent tight packing, so they remain liquid at room temp
ex: oleic acid
Polyunsaturated fat
if 2 or more double bonds are present in a hydrocarbon chain
ex: linoliec acid
Hydrogenation
→ a process used to make unsaturated fats more stable and solid at room temp by adding hydrogen to them
→occurs when some of the double bonds are removed, straightening the fat molecules so they pack more tightly, raises melting point, and makes fat more solid
→ manufacturers will use this process to make products last longer, eg. peanut butter
Cons of hydrogenation
causes trans fats, which is associated with health risks
→ it increases LDL (bad cholesterol) and lower HDL (good cholesterol), leading to increased riosk of heart disease
Triglycerides
fat molecules made up of one glycerol molecule and 3 fatty acids
→ the 3 fatty acids can be a mix of saturated, monounsaturated, and polyunsaturated types
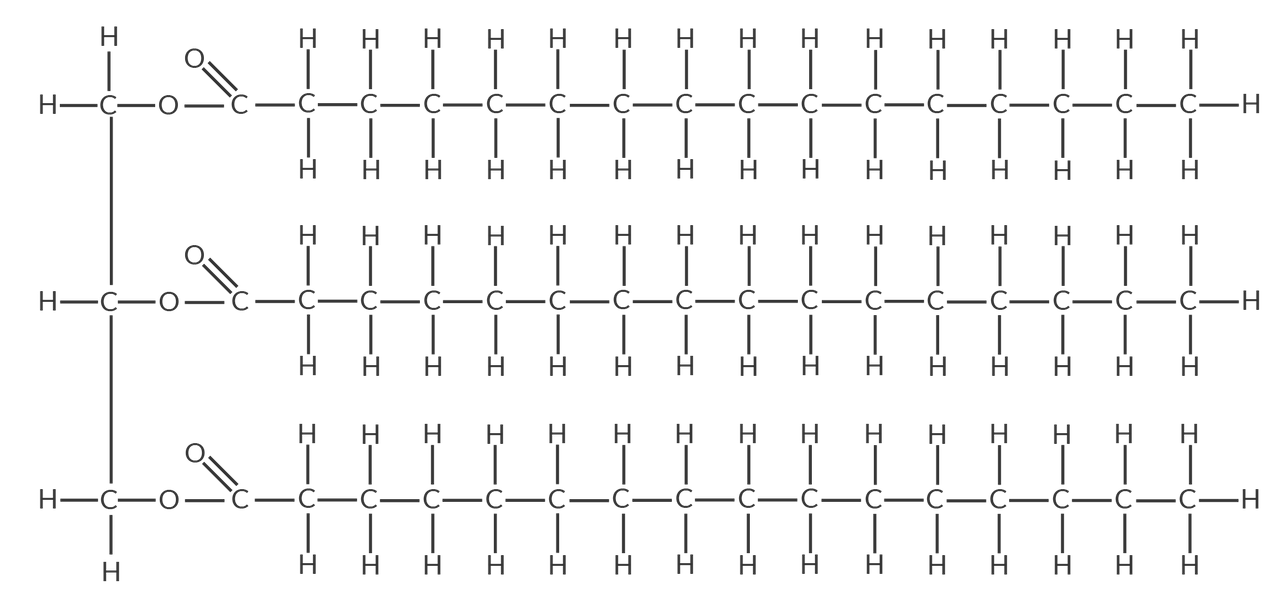
Waxes
found in products like candles, lipsticks, and polishes, and can naturally be found on leafs
→ They are malleable (eg. blend-able) and melt at a temperature over 45*C
→ It’s structure is esters of long-chain fatty acids
Steroids
another group of lipids
→ they are chemical signals that carry messages throughout the body
→ ex, testosterone→ regulates male sexual development
estradiol→ regulates portions of female sexual development
Like dissolves like
A principle stating that polar solutions will dissolve into other polar solutions, and non-polar solutions will dissolve into other non-polar solutions.
Water molecule
A molecule consisting of two hydrogen atoms and one oxygen atom (H₂O), essential to all life.
Polarity
A property of a molecule that has an uneven distribution of charges, resulting in a partial positive and negative charge.
Intermolecular force
A strong attractive force found between two molecules.
London forces
The most common form of intermolecular bond found between all atoms and molecules, They are the only way to hold non-polar molecules together
Dipole-dipole forces
Intermolecular forces found between polar molecules where positive and negative areas attract each other.
Hydrogen bond
A strong type of dipole-dipole force that occurs when hydrogen is bonded to highly electronegative atoms like N, O, or F.
Van der Waals forces
Collectively refers to London forces, dipole-dipole forces, and hydrogen bonds.
Hydrophilic
Having a liking for water; describes polar molecules and ionic substances that dissolve in water.
Hydrophobic
Having a dislike for water; describes non-polar molecules that do not easily dissolve in water.
Miscible liquid
A liquid that dissolves into another liquid.
Immiscible liquid
A liquid that forms a separate layer instead of dissolving into another liquid
Functions of proteins:
acting as biological catalysts or enzymes which enable chemical reactions in the cell to occur;
forming structural parts of organisms such as hair, tendons, fish scales, silk, etc.;
participating in cell signal and recognition factors;
acting as molecules of immunity.
Protein
natural polymers composed of long chains of amino acids linked together by amide bonds
it is a functional, three-dimensional molecule
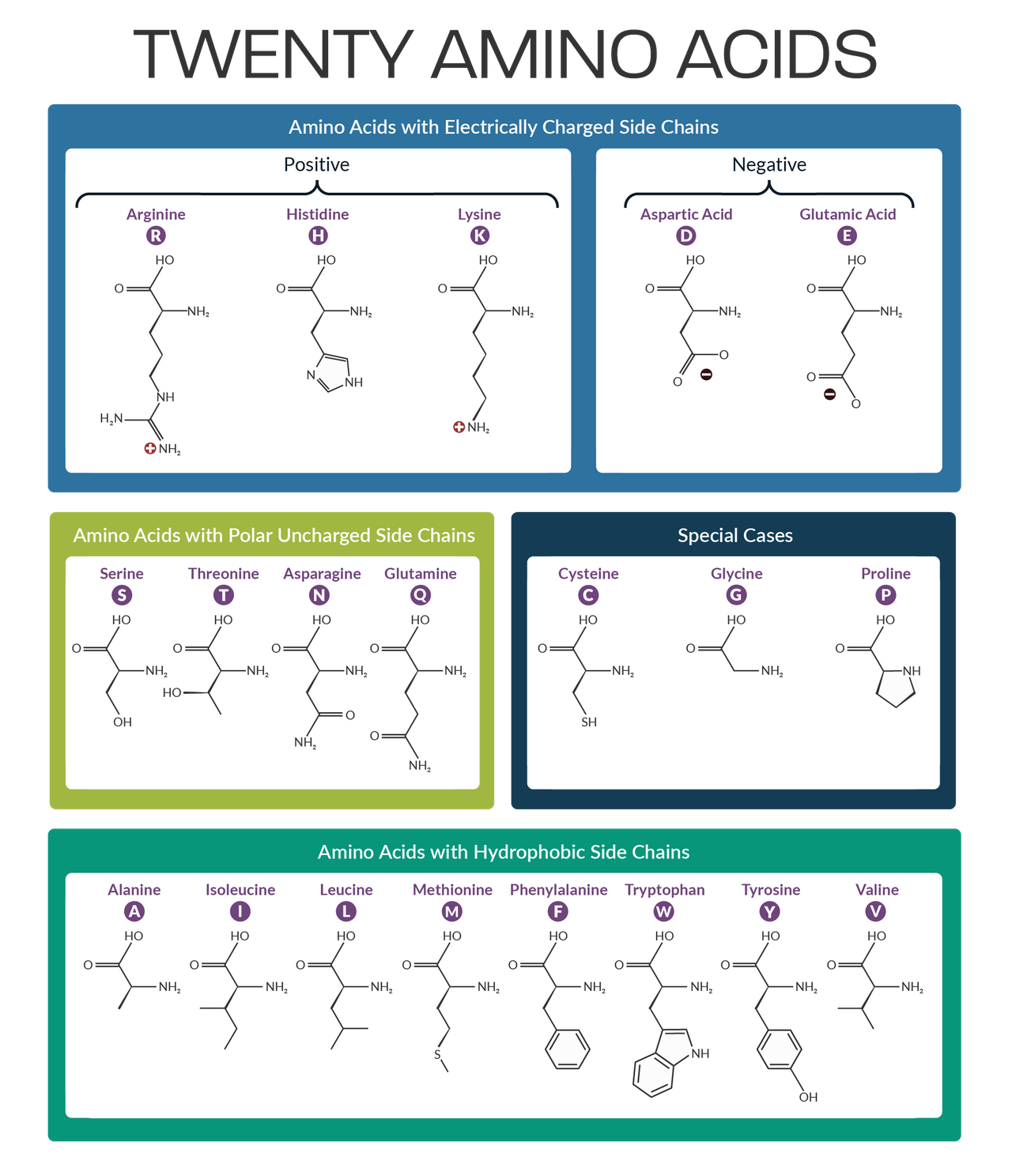
Amino acids
→the monomer subunits that polymerize into polypeptides and proteins. They consist of an amine group, a carboxyl group, and a side chain.
→The structure of the side chain determines their properties
The 20 amino acids
Dehydration synthesis
a chemical process where two molecules are joined together with the removal of a water molecule
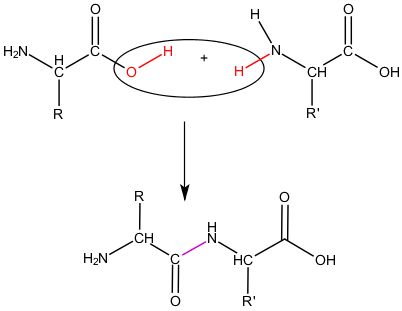
Dipeptide
Formed when two amino acid monomers are joined by dehydration synthesis
polypeptide
a large string of peptide bonds (amino acids)
Nucleic acids
are usually polymers such as proteins and carbohydrate
nucleotides
the monomer units of nucleic acids
Nucleotides are made up of three parts covalently bonded together:
a sugar
a phosphate group
and a nitrogenous base
what are the two chemical forms that nucleic acids come in?
ribonucleic acid (RNA) and deoxyribonucleic acid (DNA).
What is the difference between RNA and DNA
→RNA is normally a single-stranded polymer of nucleotides. DNA, on the other hand, is normally a double-stranded polymer of nucleotides.
→The sugars are also slightly different—RNA contains ribose sugar while DNA contains deoxyribose.
What makes up DNA?
the two strands are held together with hydrogen bonding between the bases: adenine (A) pairs with thymine (T), while cytosine (C) pairs with guanine (G). Hydrogen bonding cannot occur properly in any other combination of bases. The A and T bases share two hydrogen bonds, while C and G share three hydrogen bonds.
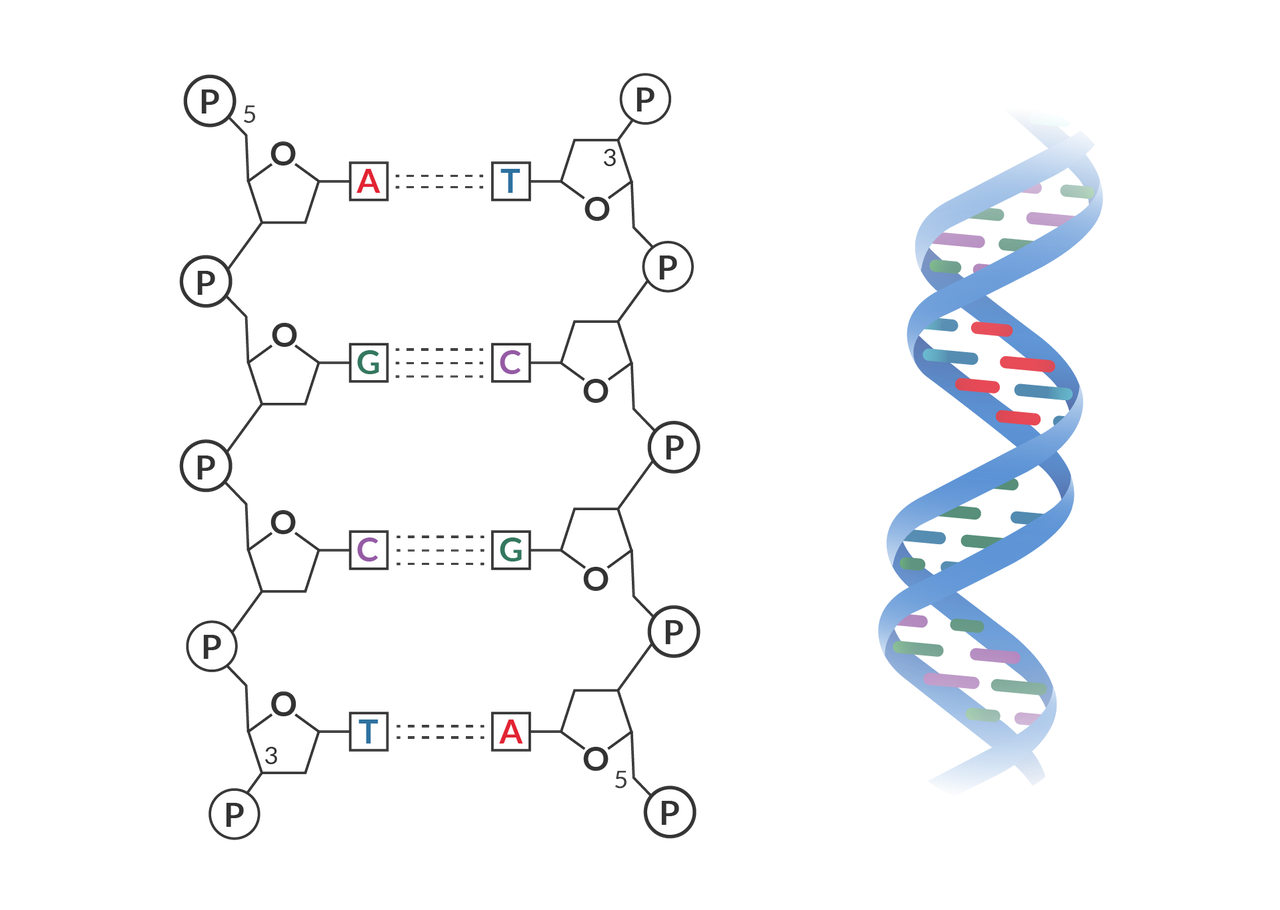
What makes up RNA?
has adenine (A), Cytosine (C), and Guanine (G). However, it does not have any thymine (T) but has uracil (U) instead. Uracil pairs with adenine
complementarity
The ability of the bases on one strand of DNA to hydrogen bond to another strand of DNA or a molecule of RNA
The cell
a discrete living unit containing all that is required for a self-propagating, independent existence and is the smallest unit that can carry on life as it fulfills all the requirements of a living system.
→they have a definite boundary
Organelles
tiny discrete functional units
→have their own architecture and independence but work with the rest of the cellular components to create life.
The cell membrane
→surrounds all the components of the cell and is composed of a double layer of lipid molecules, called a lipid bilayer.
→This layer has hydrophilic "heads" (i.e. water-loving) that are pointed out toward the exterior and interior of the cell
→hydrophobic "tails" (i.e. water-hating) that are pointed inward toward the centre of the membrane
cytoskeleton
consists of a network of structural molecules (actin filaments, intermediate filaments, and microtubules) that act as a type of scaffolding for the cell.
→It anchors organelles in place, provides shape to the cell, and is involved in movement and cell division.
cytoplasm
is the semi-fluid part of the cell that fills in the spaces around the cytoskeleton and organelles.
→ helps maintain cell shape, protects organelles, and is also involved in cellular function. Enzymes and other molecules in it break down and modify larger molecules for use by organelles.
Extracellular Fluid
This is the fluid that surrounds the outside of cells.
→composed of water and salts and helps to maintain a stable internal environment.
→It helps in the transport of fluids, oxygen, nutrients, and wastes to and from the cel
→ Examples include blood plasma and lymph, synovial fluids within joint capsules and fluid found in the eye all have specific compositions and functions.
Integral (intrinsic) proteins
permanently embedded in the phospholipid bi-layer in two ways.
They either penetrate the hydrophobic centre of the bi-layer
they stretch all of the way through the bi-layer.
Transmembrane proteins
→they stretch all of the way through the bi-layer
→come in either a globular or alpha helical shape.
The hydrophobic region of the protein is surrounded by the hydrocarbon tails of the phospholipid molecules which are also hydrophobic. Integral protiens
Peripheral (extrinsic) proteins
→are NOT permanently but loosely attached to the cell membrane's surface.
→They do not completely penetrate the bi-layer. Often they anchor themselves to exposed integral protein surfaces.
glycoproteins
when carbohydrates are bonded to embedded protiens
glycolipids
when carbohydrates are bonded to embedded lipids
Fluid Mosaic Model
→ describes the cell membrane as a flexible layer where phospholipids and proteins move freely. Phospholipids rarely flip between layers because their hydrophilic heads would pass through the hydrophobic centre.
→Membrane fluidity decreases with cold, but unsaturated phospholipids and cholesterol help maintain flexibility by preventing tight packing.
→While phospholipids form the structure, proteins in the membrane carry out most of its functions and are either integral or peripheral.
Concentration Gradient
develops as ions in solution move to greater concentration on one side of a membrane in comparison to the opposite side.
Diffusion
the movement of particles from an area of high concentration to an area of low concentration. The energy for this movement comes from the inherent motions of the particles themselves. Once the concentrations are equal, the process stops as it has entered a state of equilibrium.
Facilitated Diffusion
When large (charged) molecules or ions (charged particles) are too big to pass through the bilayer itself, they pass through embedded proteins instead.
osmosis
water molecules in relation to solute concentrations on either side of a semi-permeable membrane.
Diffuses from the least concentrated to most concentrated area
Active transport
movement of substances against their concentration gradient—from an area of low concentration to high concentration—using energy (usually from ATP).
membrane transport
A type of active transport
→Nerve cells conduct an impulse from the cell body along an axon to its terminal end where the impulse will be conducted chemically to the dendrites of the next nerve cell.
→Active transport moves sodium (Na⁺) and potassium (K⁺) across the cell membrane against their concentration gradients using energy from ATP. The Na⁺/K⁺ pump moves 3 Na⁺ out and 2 K⁺ in, creating a gradient that maintains the resting state of nerve cells.
Endocytosis
→form of active transport as it requires energy.
→The large particle is engulfed by the cell membrane forming a vesicle of fluid within the cytoplasm.
Exocytosis
the reverse of this endocytosis. Fluid-filled vesicles release waste particles by merging with the cell membrane.