Biology 302- Chapter 4: Protein Structure and Function
5.0(2)
Card Sorting
1/83
Earn XP
Description and Tags
Lecture notes put into quiz form to prep for a BIOL302 (Molecular Biology) exam.
Last updated 4:09 PM on 1/25/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
84 Terms
1
New cards
What is a structure protein? What are some examples?
It is a protein that provides the cell with shape and structure.
\
Ex: tubulin and actin
\
Ex: tubulin and actin
2
New cards
What are enzymes? What is an example?
Enzymes catalyze covalent bond breakage or formation. They are biological molecules that allow for catalytic reactions to take place.
\
Ex: pepsin
\
Ex: pepsin
3
New cards
What are transport proteins? What is an example?
Transport proteins carry other molecules or ions. They allow for movement within cells.
\
Ex: hemoglobin carrying oxygen in the bloodstream
\
Ex: hemoglobin carrying oxygen in the bloodstream
4
New cards
What are motor proteins? What is an example?
Motor proteins generate movement in cells and tissues. They function slightly differently than transport proteins.
\
Ex: Myosin
\
Ex: Myosin
5
New cards
What are storage proteins? What are some examples?
Sotrage proteins store small molecules or ions.
\
Ex: ferritin stores iron in the liver
\
Ex: ferritin stores iron in the liver
6
New cards
What are signal proteins? What is an example?
Signal proteins carry signals from cell to cell. They signal events to take place.
\
Ex: insulin signals certain glucose levels within the body
\
Ex: insulin signals certain glucose levels within the body
7
New cards
What are receptor proteins? What are some examples?
Receptor proteins detect signals and transmits them to the cell’s response machinery.
\
Example: receptors for signal proteins; insulin receptors
\
Example: receptors for signal proteins; insulin receptors
8
New cards
What are gene regulatory proteins? What are some examples?
Gene regulatory proteins bind to DNA to switch genes on or off.
\
Ex: transcriptional factors - lactose repressor
\
Ex: transcriptional factors - lactose repressor
9
New cards
What are amino acids?
They are the monomers (building blocks) that form proteins.
10
New cards
R-group and Residue are other names for a protein’s _____.
side-chain
11
New cards
Polymers are:
constructed of multiple amino acids
12
New cards
What do amino acids consist of?
Amino acids contain a central α (alpha) carbon, the n-terminus, the side chain, and the c-terminus.
13
New cards
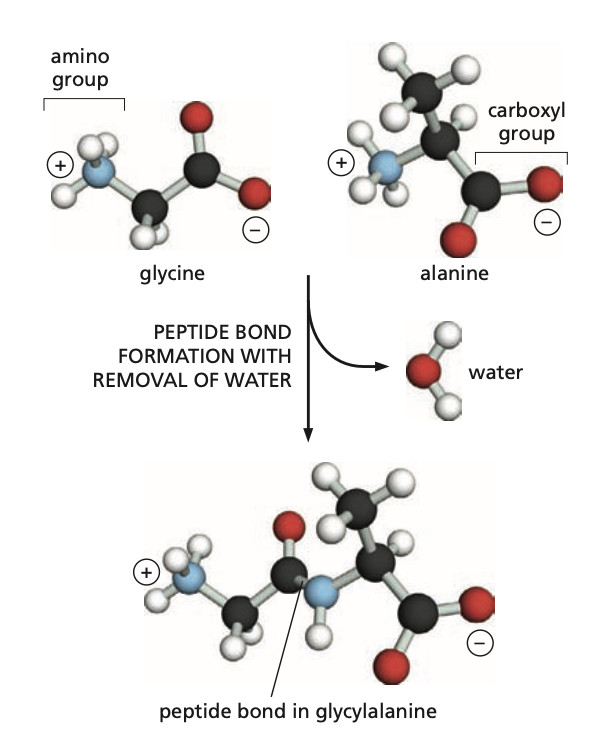
The following is an example of a ______ reaction.
condensation
14
New cards
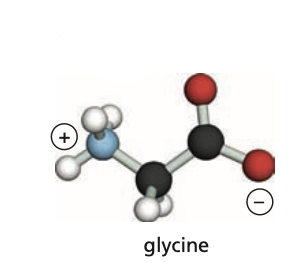
Identify the carboxyl group (C-terminus) of this protein.
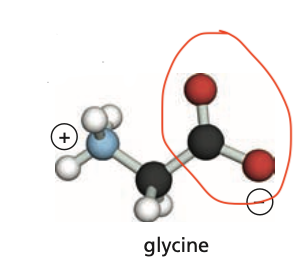
15
New cards
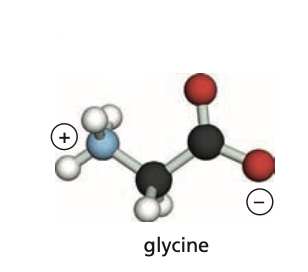
Identify the amino group (N-terminus) of this protein.
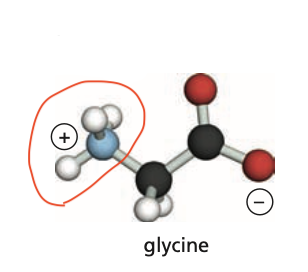
16
New cards
Polypeptides are:
constructed of multiple covalent bonds.
17
New cards
What is the terminus?
It is the end of the amino acid.
\
Examples: amino group, carboxyl group
\
Examples: amino group, carboxyl group
18
New cards
What is the peptide bond?
It is the bond between the carboxyl group from one molecule and the amino group from another molecule.
19
New cards
Whether or not an amino acid is polar or non-polar is dependent upon their:
side chain structure.
20
New cards
What are the three-letter and one-letter abbreviations for **Aspartic Acid**?
Asp; D
21
New cards
What are the three-letter and one-letter abbreviations for **Glutamic Acid**?
Glu; E
22
New cards
What are the three-letter and one-letter abbreviations for **Arginine**?
Arg; R
23
New cards
What are the three-letter and one-letter abbreviations for **Lysine**?
Lys; K
24
New cards
What are the three-letter and one-letter abbreviations for **Histidine**?
His; H
25
New cards
What are the three-letter and one-letter abbreviations for **Asparagine**?
Asn; N
26
New cards
What are the three-letter and one-letter abbreviations for **Glutamine**?
Gln; Q
27
New cards
What are the three-letter and one-letter abbreviations for **Serine**?
Ser; S
28
New cards
What are the three-letter and one-letter abbreviations for **Threonine**?
Thr; T
29
New cards
What are the three-letter and one-letter abbreviations for **Tyrosine**?
Tyr; Y
30
New cards
What are the three-letter and one-letter abbreviations for **Alanine**?
Ala; A
31
New cards
What are the three-letter and one-letter abbreviations for **Glycine**?
Gly; G
32
New cards
What are the three-letter and one-letter abbreviations for **Valine**?
Val; V
33
New cards
What are the three-letter and one-letter abbreviations for **Leucine**?
Leu; L
34
New cards
What are the three-letter and one-letter abbreviations for **Isoleucine**?
Ile; I
35
New cards
What are the three-letter and one-letter abbreviations for **Proline**?
Pro; P
36
New cards
What are the three-letter and one-letter abbreviations for **Phenylalanine**?
Phe; F
37
New cards
What are the three-letter and one-letter abbreviations for **Methinonine**?
Met; M
38
New cards
What are the three-letter and one-letter abbreviations for **Tryptophan**?
Trp; W
39
New cards
What are the three-letter and one-letter abbreviations for **Cysteine**?
Cys; C
40
New cards
What does conformation mean?
any of the spatial arrangements which the atoms in a molecule may adopt and freely convert between, especially by rotation about individual single bonds.
41
New cards
What are the three types of covalent bonds (as well as an interaction)?
* Hydrogen bonds
* Electrostatic attractions
* vaan der Waal’s attractions
* Hydrophobic interactions
* Electrostatic attractions
* vaan der Waal’s attractions
* Hydrophobic interactions
42
New cards
Even though hydrogen bonding is a fairly weak bond, multiple hydrogen bonds can add to the _____ ______ of the molecule.
structural integrity
43
New cards
Prion diseases are caused by:
rare proteins whose mis-folding is infectious.
44
New cards
What is a heterodimer?
Two different proteins interacting.

45
New cards
What is a homodimer?
Two of the same proteins interacting.

46
New cards
___________ can guide the folding of a newly synthesized polypeptide chain.
Chaperone proteins
47
New cards
What are the levels of protein organization?
* Primary
* Secondary
* Tertiary
* Quaternary
* Secondary
* Tertiary
* Quaternary
48
New cards
What does the primary structure of a protein refer to?
The linear amino acid shape/structure/sequence of the protein
49
New cards
What does the secondary structure of a protein refer to?
An organization that forms within certain segments of the polypeptide chain; Specific folding patterns (motifs)
50
New cards
What does the tertiary structure of a protein refer to?
The full, three dimensional conformation of the entire polypeptide; The fully folded form of protein
51
New cards
What does the quaternary structure of a protein refer to?
a complex of more than one polypeptide joined together
52
New cards
What is the polypeptide backbone?
The polypeptide backbone is the key contributor to protein secondary structure, which involves backbone-to-backbone hydrogen bonding
53
New cards
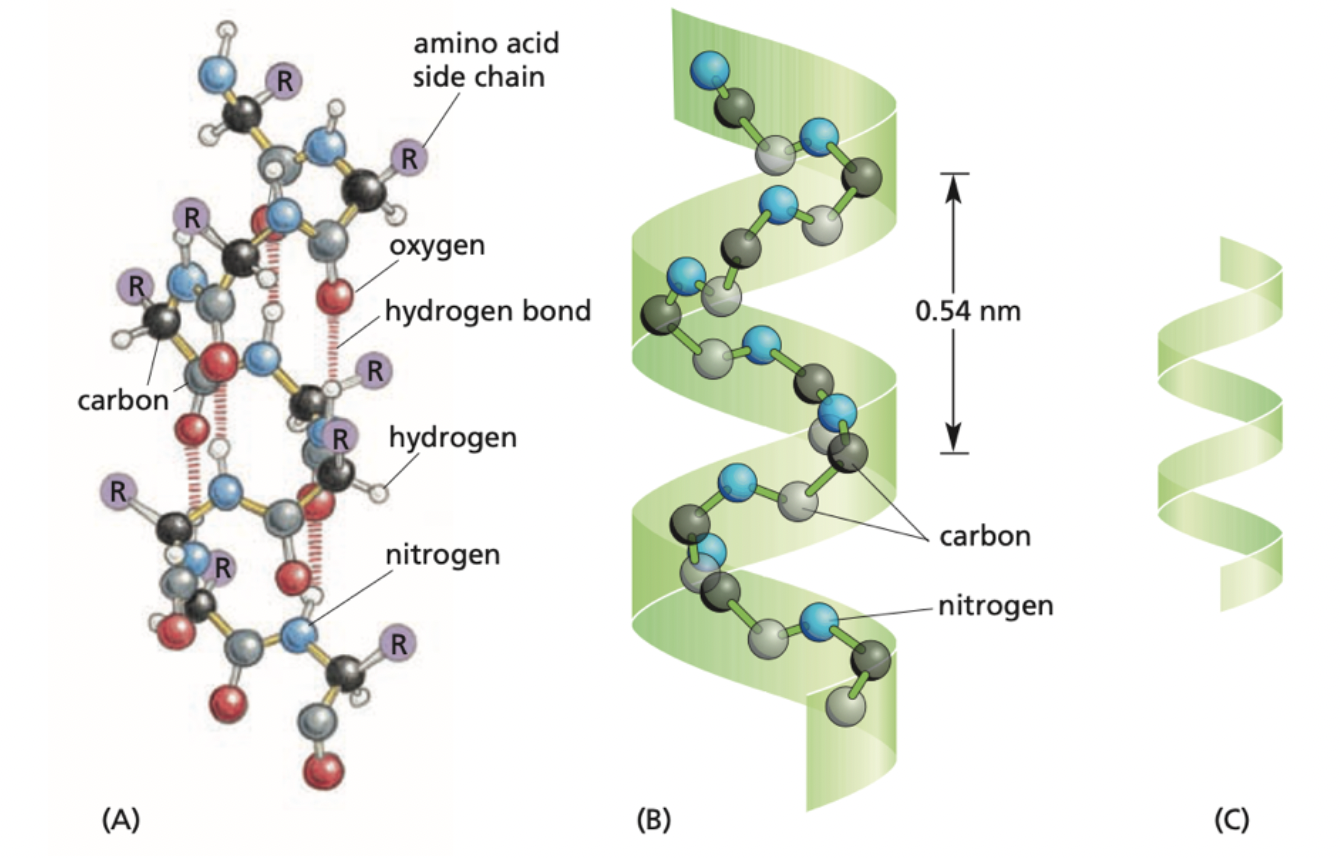
What is this an image of?
The alpha helix folding pattern is demonstrated in a linear sequence of amino acids that folds in a helical manner that is stabilized internally through hydrogen bonds between the polypeptide backbone.
54
New cards
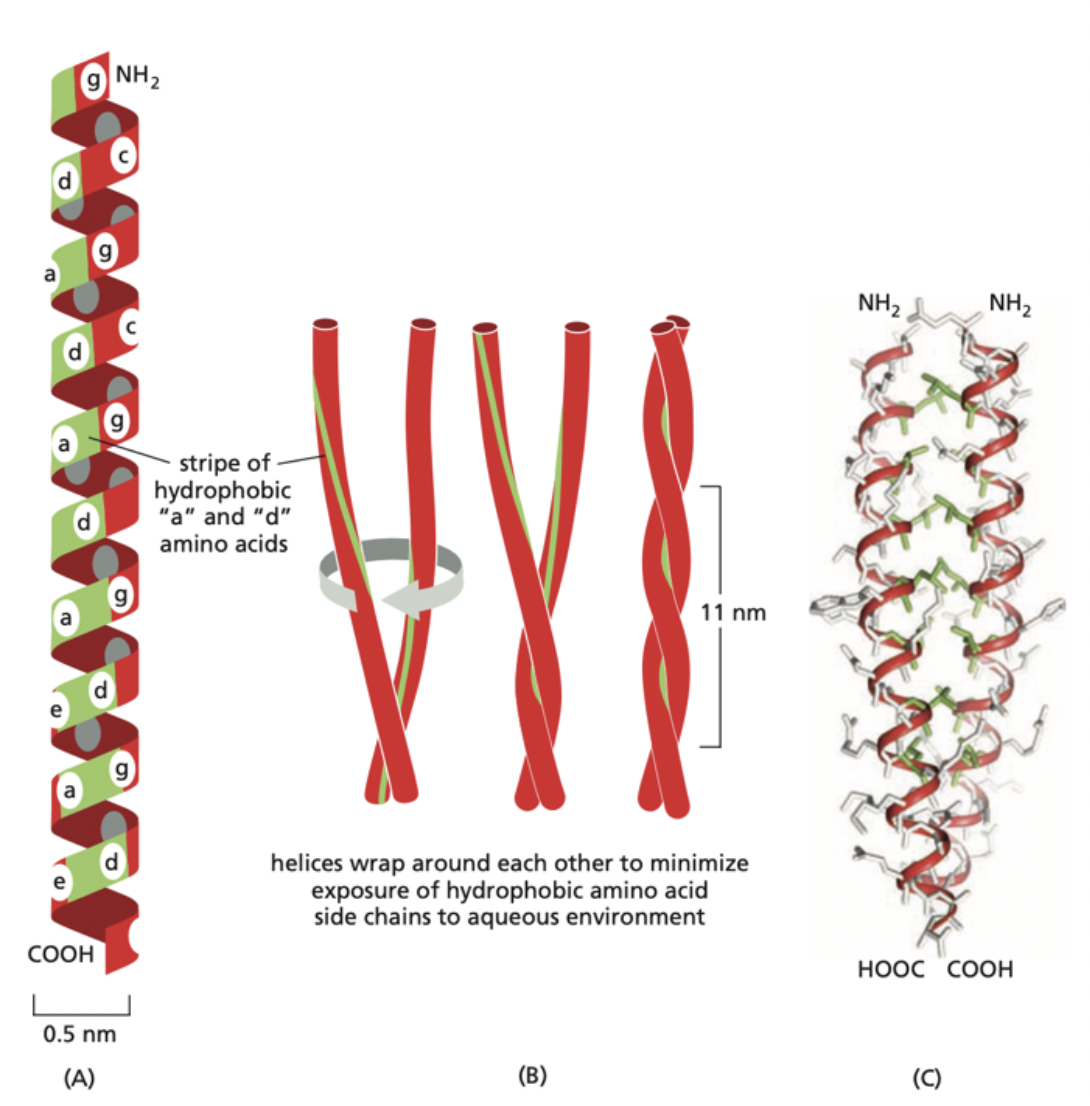
What is this an image of?
When alpha helices interact and intertwine with each other. When they do this, it is referred to as a “coiled-coil” interaction. The helices do this coiled-coil arrangement in order to minimize the exposure of their hydrophobic residues to their surrounding aqueous environment.
55
New cards
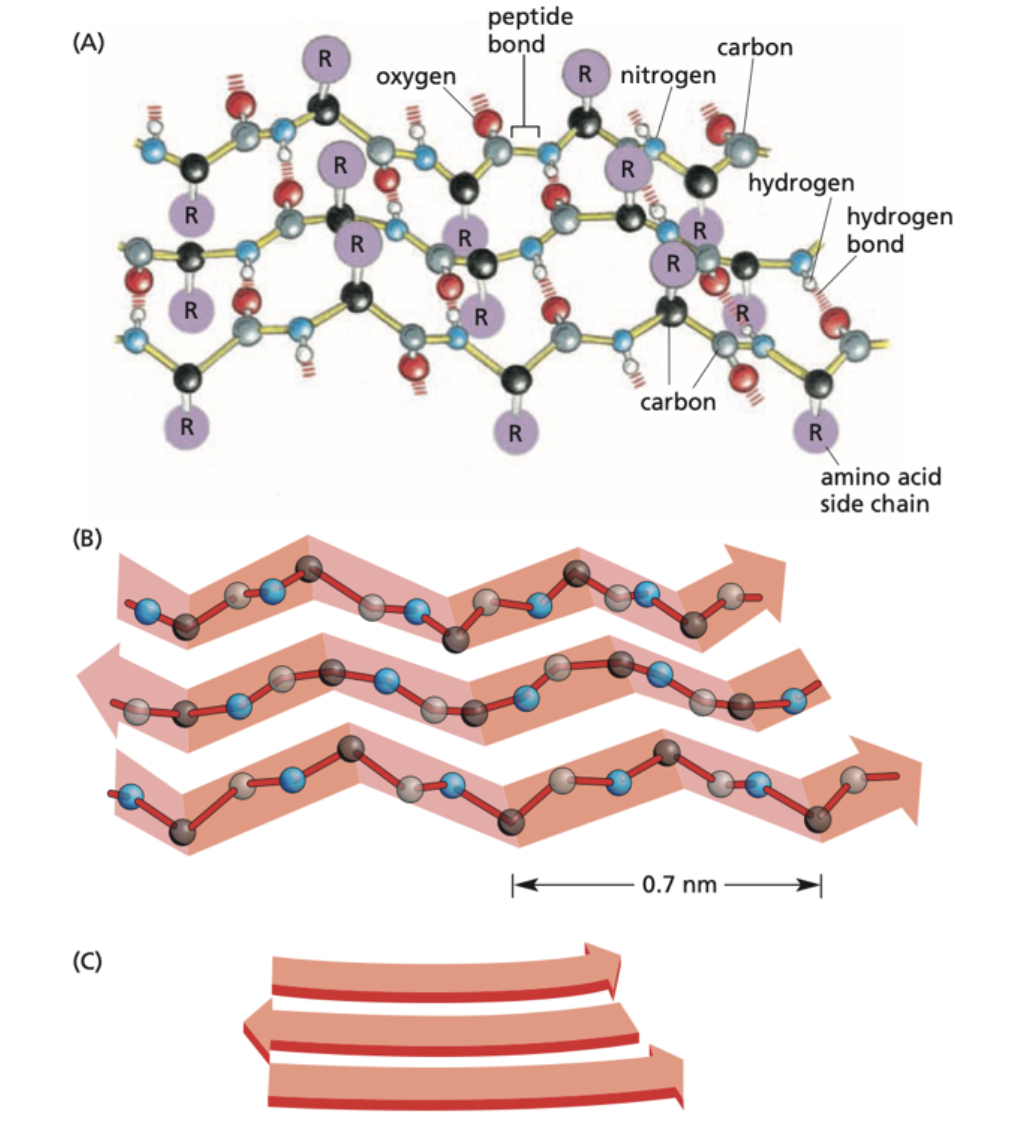
What is this an image of?
The β-sheet folding pattern. It involves neighboring regions of the polypeptide chain that are associated side-by-side through hydrogen bonding and give it a flat/rigid structure.
56
New cards
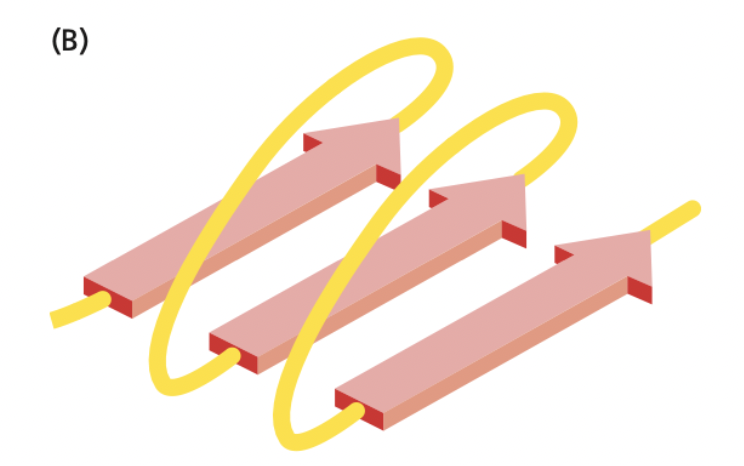
What is this an image of?
A β-sheet variety called parallel. It is where neighboring polypeptide chains run in the same orientation
57
New cards
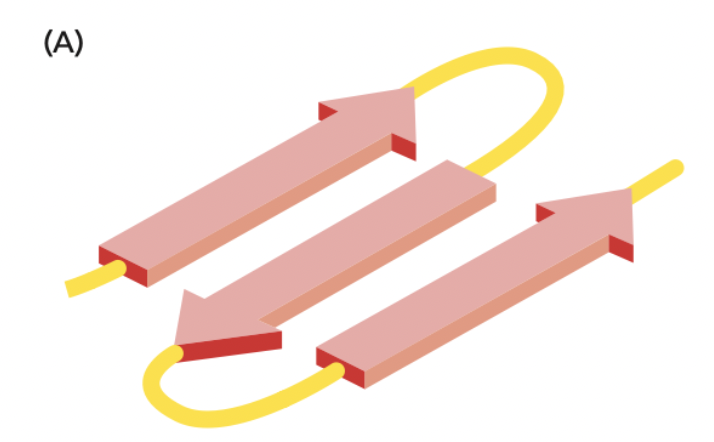
What is this an image of?
A β-sheet variety called anti-parallel. It is where neighboring polypeptide chains run in opposite direction.
58
New cards
What is a functional domain?
Domains are distinct functional and/or structural units in a protein. Usually they are responsible for a particular function or interaction, contributing to the overall role of a protein.
59
New cards
Proteins can assemble into:
filaments, sheets, or spheres.
60
New cards
The orientation in which a protein forms is affected by:
the number of binding sites each protein contains.
61
New cards
If a protein has only one binding site, it can form a _____ with another identical protein.
dimer
62
New cards
Identical proteins with 2 binding sites will often form a long _____ *___*__.
helical filament
63
New cards
If the two identical proteins with two binding sites are oriented properly, the proteins can also form a ______.
sphere/ring
64
New cards
Single protein subunits can pack to form a:
filament, tube, or spherical shell.
65
New cards
______ _______ help stabilize a favored protein conformation.
Disulfide bonds
66
New cards
Proteins often form large complexes that function as machines. Many interacting proteins are brought together by:
scaffold proteins
67
New cards
What is a ligand?
Any molecule that can bind to another protein
68
New cards
If the binding of the protein and the ligand is unfavored or has poorly matching surfaces, the two molecules will:
dissociate (pull apart).
69
New cards
What is feedback inhibition?
When an enzyme that acts early in a pathway is inhibited by a late product of the pathway. Feedback inhibition regulates the flow through biosynthetic pathways. In some occasions, it can trigger a conformational change in the protein.
70
New cards
What are the nucleoside triphosphates?
ATP (Adenosine Tri-Phosphate)
CTP (Cytidine Tri-Phosphate)
TTP (Thymidine Tri-Phosphate)
GTP (Guanosine Tri-Phosphate)
CTP (Cytidine Tri-Phosphate)
TTP (Thymidine Tri-Phosphate)
GTP (Guanosine Tri-Phosphate)
71
New cards
The binding of a regulatory ligand can change the _______ between two protein conformations.
equilibrium
72
New cards
Protein ______ is a very common means of regulating protein activity.
phosphorylation
73
New cards
The enzyme involved in the removal of a phosphate group is called:
phosphatase
74
New cards
GTP-binding proteins form:
molecular switches.
75
New cards
The modification of a protein at multiple sites produce a regulatory code that controls the:
protein behavior.
76
New cards
K! 1) How many different amino acids are used in making proteins?
20
77
New cards
K! 2) The phosphorylation of a protein is typically associated with a change in activity, the assembly of a protein complex, or the triggering of a downstream signaling cascade. The enzyme that catalyzes the addition of a phosphate group onto a protein is called a:
kinase
78
New cards
K! 3) Which of the following is *not* a feature commonly observed in β sheets?
\
a. anti-parallel regions
b. coiled-coil structure
c. parallel regions
d. extended polypeptide backbone
\
a. anti-parallel regions
b. coiled-coil structure
c. parallel regions
d. extended polypeptide backbone
coiled-coil structure
79
New cards
K! 4) In a folded protein, most of the nonpolar amino acids are buried inside the protein fold, whereas the polar and charged side chains are exposed to the components in the cytosol. This fold is more stable because of the expulsion of non-polar atoms from contact with water, favoring the interaction of non-polar atoms with each other. What is this type of non-covalent interaction called?
hydrophobic interaction
80
New cards
K! 5) The correct folding of proteins is necessary to maintain healthy cells and tissues. Abnormally folded proteins can be responsible for such neurodegenerative disorders as Alzheimer’s, Huntington’s, and Creutzfeld–Jacob disease (the specific faulty protein is different for each disease). What is the ultimate fate of these disease-causing, abnormally folded proteins?
Incorrectly folded proteins can develop into protein aggregates with an amyloid structure and damage cells or tissues.
81
New cards
K! 6) Why are α helices and β sheets common folding patterns in polypeptides?
Because the bonds involved are hydrogen bonds, not the side-chain.
82
New cards
K! 7) What does the primary structure of a protein refer to?
The amino acid sequence of the protein.
83
New cards
K! 8) What are protein families?
Evolutionarily related proteins that are similar in amino acid sequence and 3D shape.
84
New cards
K! 9) Protein molecules that have a quaternary structure have:
two or more polypeptide chains.