how to draw bohr rutherford, bohr and lewis diagrams
1/3
Earn XP
Description and Tags
bohr ruther ford 18/2/25,
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
positioning of electrons
at 3
at 6
at 9
at 12
always fill first 4 positions before starting pairs
for Helium, draw the two as a pair (together) at the top or right
in the first orbit, the two electrons will always be a pair at the top or right
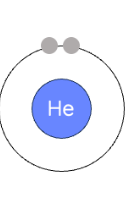
how to draw bohr rutherford diagram looking at periodic table
period # = # of shells
group # = # of valence electrons (groups 1-8)
first orbit : max 2 pairs
all other orbits: max 18 electrons, 4 pairs
nucleus: # P and # N written
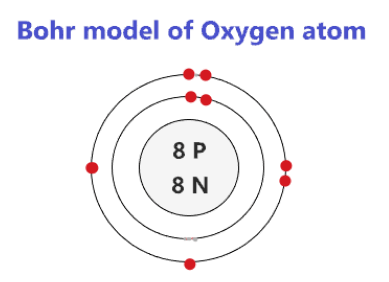
how to draw bohr diagram looking at periodic table
period # = # of shells
group # = # of valence electrons (groups 1-8)
first orbit : max 2 pairs
all other orbits: max 18 electrons, 4 pairs
nucelus: no cricle around it, just element symbol
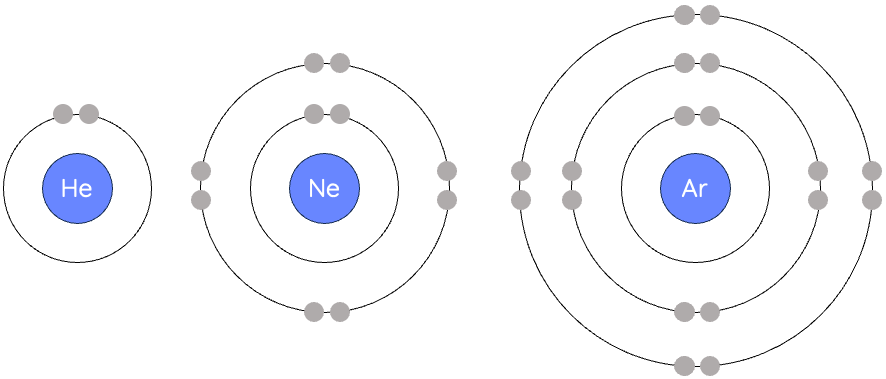
how to draw lewis diagrams
only includes valence electrons
He is the only one where e is in a pair on the right, all other elements have them separated (fill first 4 positions before starting pairs)
elements name in middle