Lecture 16: Ionotropic Receptors
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
66 Terms
What are the 2 types of NT receptors?
Ionotropic: Rapid action, short-term effect, signaling (ligand-gated)
GPCR: Slow onset, longer effect, modulatory
What determines whether an ionotropic receptor causes depolarization or hyperpolarization?
If the receptor is a cation channel (e.g., Na⁺, K⁺, Ca²⁺), the result is depolarization → EPSP.
If the receptor is a Cl⁻ channel, the result is often hyperpolarization → IPSP.
How many subunits make up the major classes of ionotropic receptors?
5 subunits: ACh (nicotinic), GABAA, glycine, and 5-HT receptors.
4 subunits: Glutamate receptors (AMPA, NMDA, kainate).
3 subunits: Ionotropic ATP receptors, each subunit with 2 TM domains.
What is the structure of each ionotropic glutamate receptor subunit?
Each subunit has three transmembrane helices (M1, M3, M4) and one pore loop (M2) that dips into the membrane from the cytoplasmic side.
What determines the ion selectivity of glutamate receptors?
The M2 pore loop determines the receptor’s cation permeability.
Where is the glutamate binding site located?
It is formed by the N-terminal domain and the extracellular loop between the 3rd and 4th TM helices.
What channel type do ionotropic glutamate receptors structurally resemble?
They resemble K⁺ channels, but their pore loop orientation is inverted — it forms from the cytoplasmic side.
What are the 3 major classes of ionotropic glutamate receptors and what subunits do they contain?
NMDA (GluN1-3), AMPA (GluA1-4), and Kainate (GluK1-5) receptors.
What role does the GluR2 (GluA2) subunit play in AMPA receptors?
The GluR2 subunit eliminates Ca²⁺ permeability and reduces current flow through the receptor channel.
What is the Q/R site in AMPA receptors?
The Q/R site is a position in the pore loop (M2) of AMPA receptor subunits that determines Ca²⁺ permeability.
What amino acid is found at the Q/R site in GluR2 subunits, and what effect does it have?
Contains an arginine (R) at the Q/R site, which blocks Ca²⁺ influx, making the receptor impermeable to Ca²⁺.
What amino acid is found at the Q/R site in GluR1, GluR3, and GluR4 subunits?
Contain glutamine (Q) at the Q/R site, allowing the channel to be Ca²⁺-permeable.
How is the arginine (R) residue at the Q/R site of GluR2 generated?
It is created through RNA editing of the GluR2 mRNA, where a CAG (glutamine) codon is converted to CIG
What enzyme performs the RNA editing at the GluR2 Q/R site?
ADAR2 (adenosine deaminase acting on RNA type 2
Why is RNA editing at the Q/R site of GluR2 important?
Editing introduces a positively charged arginine residue in the channel pore, which blocks Ca²⁺ permeability.
What happens in ADAR2 or GluR2 mutants lacking this RNA edit?
They express AMPA receptors with glutamine (Q) instead of arginine (R), making them Ca²⁺-permeable. Such neurons have excessive conductance and can cause fatal seizures.
What is the role of polyamine block in AMPA receptors?
Polyamines can temporarily block non-GluR2 AMPA channels, reducing cation flow. This block is activity-dependent and can be relieved by repeated activation, allowing Ca²⁺ influx that contributes to synaptic plasticity.
What makes NMDA receptors unique among ionotropic glutamate receptors?
NMDA receptors are both ligand-gated and voltage-dependent, requiring glutamate binding and membrane depolarization to open.
They are coincidence detectors.
Which ions can pass through NMDA receptors?
Na+, K+, Ca2+
What co-agonists are required for NMDA receptor activation?
Either D-serine or glycine must bind along with glutamate for the channel to open.
What drugs or molecules interact with the NMDA receptor channel?
Mg²⁺ blocks the channel at rest (voltage-dependent block).
Memantine is an uncompetitive antagonist that binds the Mg²⁺ site to prevent excessive activation.
D-serine, released by astrocytes, acts as a co-agonist at a separate binding site.
What does the I–V curve of NMDA receptors show about Mg²⁺’s role?
In the presence of Mg²⁺, little current flows at hyperpolarized potentials because Mg²⁺ blocks the pore. When the cell depolarizes, the Mg²⁺ block is removed, allowing outward, linear current flow.
What happens to NMDA receptor current in the absence of Mg²⁺?
The current–voltage (I–V) relationship becomes linear, with inward current at negative potentials (cation entry) and outward current at positive potentials — showing that NMDA channels are non-selective cation channels.
Why is the current reversal point near 0 mV?
Because NMDA receptors allow Na⁺, K⁺, and Ca²⁺ to flow freely, and at 0 mV the electrochemical driving forces for these ions balance out, resulting in no net current.
What is the functional consequence of Mg²⁺ block in NMDA receptors?
It makes the channel voltage-dependent, allowing NMDA receptors to act as coincidence detectors.
What is an NMDA-R antagonist?
APV
What is an AMPA-R antagonist?
CNQX
How do AMPA and NMDA receptors differ in their current–voltage (I–V) relationship?
AMPA receptors (especially those with GluA2) show a linear I–V curve — current flows inward below 0 mV and outward above 0 mV, reversing at ~0 mV.
NMDA receptors show a nonlinear I–V curve due to Mg²⁺ block at hyperpolarized potentials; current increases only when the membrane depolarizes enough to remove the block.
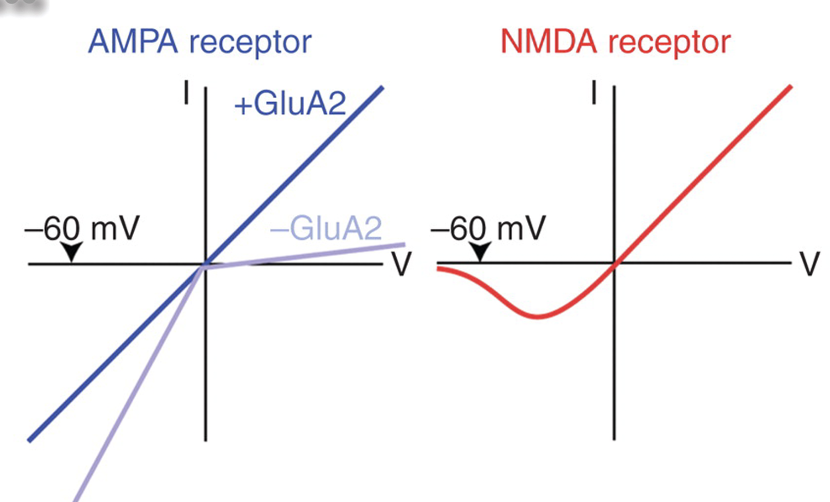
What causes the Mg²⁺ block in NMDA receptors?
At resting (hyperpolarized) potentials, Mg²⁺ ions sit inside the NMDA receptor pore, preventing ion flow. When the membrane depolarizes, Mg²⁺ is expelled by electrostatic repulsion, allowing Na⁺ and Ca²⁺ to enter.
What are the 5 types of proteins in the glutamate postsynaptic density (PSD)?
1) Receptors
2) Scaffolding proteins
3) Adhesion molecules
4) Regulatory (effector) proteins
5) Cytoskeletal proteins
What 2 scaffolding proteins organize the PSD?
PSD-95 and Shank form two interconnected scaffolding layers.
How are NMDA and AMPA receptors anchored in the PSD?
NMDA and kainate receptors bind directly to PSD-95.
AMPA receptors are linked indirectly via GRIP (glutamate receptor–interacting protein).
What connects the two scaffolding layers and anchors them to the cytoskeleton?
GKAP links PSD-95 and Shank
Cortactin binds Shank to F-actin.
What is the role of mGluRs and Homer in the PSD?
mGluRs (metabotropic glutamate receptors) at the synapse edge are tethered to Shank by Homer proteins.
Why are scaffolding proteins important?
They hold effector proteins like nNOS and CaMKII near NMDA receptors, ensuring Ca²⁺ influx via NMDA-Rs triggers localized signaling — such as nitric oxide (NO) production and synaptic plasticity.
What two proteins link the pre- and postsynaptic membranes?
Adhesion proteins β-neurexin (presynaptic) + neuroligin (postsynaptic) form a bridge across the synaptic cleft to align the two sides of the synapse.
What do β-neurexin and neuroligin bind to inside each neuron?
β-neurexin binds to CASK, a presynaptic scaffolding protein.
Neuroligin binds to PSD-95, a postsynaptic scaffolding protein.
What are silent synapses?
Glutamate synapses that contain only NMDA receptors.
Since NMDA-R blocked by Mg2+ at resting potential, no EPSP produced when presynaptic axon releases NT.
How can a silent synapse be “unsilenced”?
Insertion of AMPA-R into postsynaptic membrane.
What does “cycling” of AMPA receptors mean?
AMPA receptors can move in and out of the postsynaptic membrane, allowing synapses to switch between silent (inactive) and functional (active) states.
Why are silent synapses important?
They are key to synaptic plasticity and learning, as AMPA receptor insertion during LTP (long-term potentiation) transforms silent synapses into active ones.
What is glutamate excitotoxicity?
Neuronal death caused by excessive activation of glutamate receptors (especially extrasynaptic NMDA-R and VgCCs).
What’s the difference between synaptic and extrasynaptic NMDA receptor activation?
Synaptic NMDARs → trigger survival and plasticity pathways
Extrasynaptic NMDARs → trigger cell death pathways
What causes excessive glutamate release and excitotoxicity?
Conditions like stroke, ischemia, or oxygen deprivation cause uncontrolled glutamate release and sustained activation of NMDA and voltage-gated Ca²⁺ channels.
What type of receptor is the nicotinic acetylcholine receptor (nAChR)?
Pentameric ligand-gated ion channel (PLGIC) made of 5 subunits (2α, 1β, 1γ, 1δ) that form a central pore for ion flow.
How many TM helices does each nAChR subunit have, and which one lines the pore?
Each subunit has 4 transmembrane helices (TM1–TM4). TM2 from each subunit lines the pore of the channel.
Where is the ligand (acetylcholine) binding site located?
Extracellular N-terminus of the subunits forms the ligand-binding site.
How does acetylcholine binding open the channel?
Binding of ACh causes a conformational change that:
1) Rotates TM2, moving blocking leucine (large, nonpolar) residues out of the way.
2) Aligns polar amino acids (serine, threonine) to open the pore for ion passage.
What type of ions flow through nAChR channels?
Mainly Na⁺ and K⁺, allowing depolarization of the postsynaptic membrane.
What type of ions can pass through the nAChR channel?
The nAChR is a non-selective cation channel, allowing Na⁺, K⁺, and Ca²⁺ to flow, but excluding Cl⁻.
How does the nAChR achieve selectivity for cations over anions?
It has 3 rings of negatively charged amino acids. Attract positive ions, repel negative.
What type of ion channel is the GABAₐ receptor?
It’s a Cl⁻ (chloride) channel, allowing influx of Cl⁻ ions, which typically hyperpolarizes the postsynaptic neuron.
How is the structure of the GABAₐ receptor similar to the nAChR?
Both are pentameric ligand-gated ion channels (PLGICs) — made of five subunits, each with four transmembrane helices (M1–M4), arranged around a central pore.
How do GABAₐ receptor subunits differ from nAChR subunits?
GABAₐ receptor subunits form an anion channel for Cl⁻.
Have positively charged amino acid rings (e.g., lysine, arginine) lining the pore
What are common binding sites on the GABAₐ receptor?
GABA site → binds NT GABA to open the channel
Benzodiazepine site → enhances GABA binding (e.g., diazepam)
Barbiturate & anesthetic sites → modulate receptor activity (e.g., propofol, ethanol)
Can a cation-selective channel be converted into an anion-selective one?
Yes — by mutating just three key amino acids in the M2 pore region of AChRα7 you can convert it into an inhibitory receptor.
What does the M2 domain do?
Forms the ion-selective pore of ligand-gated ion channels, and its amino acid composition determines whether the channel passes cations or anions.
What are allosteric binding sites on the GABAₐ receptor?
Secondary binding sites located away from the main GABA binding site where other molecules, like barbiturates or benzodiazepines (e.g., Valium), can bind.
What happens when a drug binds to an allosteric site on the GABAₐ receptor?
The drug does not directly open the channel, but instead enhances the effect of GABA.
Why does GABAₐ receptor activation cause depolarization in developing neurons but hyperpolarization in mature ones?
In developing neurons, the Na⁺-K⁺-2Cl⁻ cotransporter (NKCC1) keeps intracellular Cl⁻ levels high. When GABAₐ channels open, Cl⁻ flows out, causing depolarization.
In mature neurons, the K⁺-Cl⁻ cotransporter (KCC2) lowers intracellular Cl⁻, so Cl⁻ flows in when GABAₐ opens, leading to hyperpolarization (inhibition).
What molecular switch changes GABA’s effect during development?
The downregulation of NKCC1 and upregulation of KCC2.
shifts Cl⁻ flow from outward (depolarizing) to inward (hyperpolarizing).
What protein anchors GABAₐ and glycine receptors to the cytoskeleton?
Gephyrin.
Scaffolding protein, linking receptors to underlying actin filaments and microtubules.
What are the two main types of GABA receptors and how do they differ?
GABAₐ receptors are ionotropic:
Cl⁻ channels that open directly when GABA binds, causing fast inhibitory postsynaptic potentials (IPSPs).
GABA_B receptors are GPCRs
Work through G-proteins (Gᵢ) to open K⁺ channels and close Ca²⁺ channels, producing slower, longer-lasting inhibition.
What is the effect of activating GABA_B receptors?
Activation increases K⁺ conductance (hyperpolarizing the neuron) and decreases Ca²⁺ conductance, reducing NT release.