Orgo Exam 1
1/110
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
111 Terms
Alcohol

Aldehyde
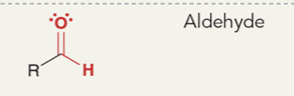
Alkane
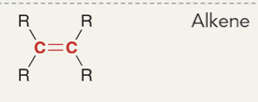
Alkene
Alkyl Halide

Alkyne

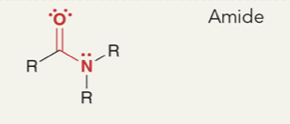
Amide
Amine

Aromatic Compound

Carboxylic Acid

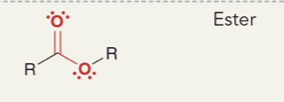
Ester
Ether

Ketone

Acyl halide

Sulfide

Thiol

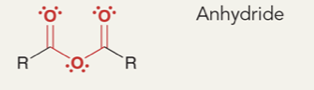
Anhydride
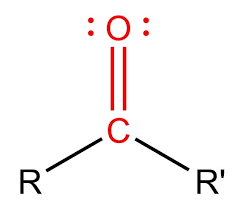
Carbonyl
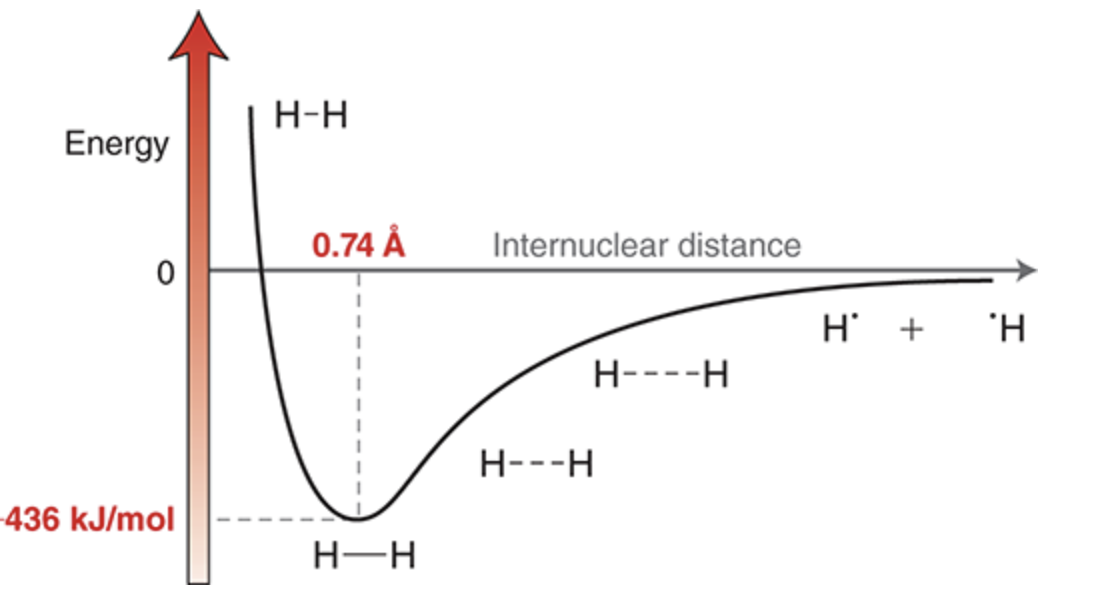
Energy during formation of a bond
Moving toward the left on the diagram, the hydrogen atoms approach each other, and there are several forces that must be taken into account: (1) the force of repulsion between the two negatively charged electrons, (2) the force of repulsion between the two positively charged nuclei, and (3) the forces of attraction between the positively charged nuclei and the negatively charged electrons.
This provides for a net force of attraction, which lowers the energy of the system. As the hydrogen atoms move still closer together, the energy continues to be lowered until the nuclei achieve a separation (internuclear distance) of 0.74 angstroms (Å).
At that point, the force of repulsion between the nuclei begins to overwhelm the forces of attraction, causing the energy of the system to increase if the atoms are brought any closer together. The lowest point on the curve represents the lowest energy (most stable) state. This state determines both the bond length (0.74 Å) and the bond strength (436 kJ/mol)
Identifying a formal charge
Determine the appropriate number of valence electrons for an atom.
Determine whether the atom exhibits the appropriate number of electrons.
For our purposes, we must split each bond apart equally, and then count the number of electrons on each atom.
EN cutoffs for bond strength
If the difference in electronegativity is less than 0.5, the electrons are considered to be equally shared between the two atoms, resulting in a covalent bond.
If the difference in electronegativity is between 0.5 and 1.7, the electrons are not shared equally between the atoms, resulting in a polar covalent bond.
If the difference in electronegativity is greater than 1.7, the electrons are not shared at all.
How to draw dipole moment
Partial charges resulting from induction
Identify all polar covalent bonds.
Determine the direction of each dipole.
Draw net dipole
Rules for bond line structures
The two carbon atoms of a triple bond and the two carbon atoms connected to them are drawn in a straight line. All other bonds are drawn in a zigzag format
Hydrogen atoms bonded to carbon are also not shown in bond-line structures, because it is assumed that each carbon atom will possess enough hydrogen atoms so as to achieve a total of four bonds (an octet of electrons).
Principles for filling orbitals
The Aufbau principle. The lowest energy orbital is filled first.
The Pauli exclusion principle. Each orbital can accommodate a maximum of two electrons that have opposite spin.
Hund’s rule. When dealing with degenerate orbitals, such as p orbitals, one electron is placed in each degenerate orbital first, before electrons are paired up.
Constructive and destructive interference
Constructive interference produces a wave with larger amplitude. In contrast, destructive interference results in waves canceling each other, which produces a node
Valence bond theory
According to valence bond theory, a bond is simply the sharing of electron density between two atoms as a result of the constructive interference of their atomic orbitals.
The electron density of this bond is primarily located on the bond axis (the line that can be drawn between the two hydrogen atoms). This type of bond is called a sigma (σ) bond and is characterized by circular symmetry with respect to the bond axis.
Molecular orbital theory
According to this theory, atomic orbitals are mathematically combined to produce new orbitals, called molecular orbitals.
Both types of orbitals are used to accommodate electrons, but an atomic orbital is a region of space associated with an individual atom, while a molecular orbital is associated with an entire molecule. That is, the molecule is considered to be a single entity held together by many electron clouds, some of which can actually span the entire length of the molecule.
electrons first occupy the lowest energy orbitals, with a maximum of two electrons per orbital.
The lower energy molecular orbital, or bonding MO, is the result of constructive interference of the original two atomic orbitals. The higher energy molecular orbital, or antibonding MO, is the result of destructive interference. Notice that the antibonding MO has one node, which explains why it is higher in energy. Both electrons occupy the bonding MO in order to achieve a lower energy state. This lowering in energy is the essence of the bond.
Constitution of hybrid orbitals
This procedure gives us four orbitals that were produced by averaging one s orbital and three p orbitals, and therefore we refer to these atomic orbitals as sp3-hybridized orbitals.
They are called sp2-hybridized orbitals to indicate that they were obtained by averaging one s orbital and two p orbitals.A triple bond is formed by sp-hybridized carbon atoms. To achieve sp hybridization, one s orbital is mathematically averaged with only one p orbital
This leaves two p orbitals unaffected by the mathematical operation. As a result, an sp-hybridized carbon atom has two sp orbitals and two p orbitals
VSEPR theory
The shapes of small molecules can often be predicted if we presume that all electron pairs (whether bonding or nonbonding) repel each other, and as such, they arrange themselves in three-dimensional space so as to achieve maximal distance from each other.
Steric number
The total of electron pairs (single bonds + lone pairs) for an atom in a compound.
Lone pairs in VSEPR theory
This shorter bond angle can be justified by VSEPR theory if we presume that lone pairs repel more strongly than σ bonds (lone pairs are not bound by another nucleus, so they occupy more space than bonding electron pairs).
Dipole moment
The dipole moment (μ) is used as an indicator of polarity, where μ is defined as the amount of partial charge (δ) on either end of the dipole multiplied by the distance of separation (d)
Measuring the dipole moment of a particular bond allows us to calculate the percent ionic character of that bond.
The vector sum is called the molecular dipole moment, and it takes into account both the magnitude and the direction of each individual dipole moment.
Hydrogen bonding
When a hydrogen atom is connected to an electronegative atom (usually O or N), the hydrogen atom will bear a partial positive charge (δ+) as a result of induction. This δ+ can then interact with a lone pair from an electronegative atom of another molecule or ion.
This type of interaction is quite strong because hydrogen is a relatively small atom, and as a result, the partial charges can get very close to each other. In fact, the effect of hydrogen bonding on physical properties is quite dramatic
LDF trends
Specifically, the boiling point appears to increase with increasing molecular weight. This trend can be justified by considering the fleeting, or transient, dipole moments that are more prevalent in larger hydrocarbons.
Large hydrocarbons have more surface area than smaller hydrocarbons and therefore experience these attractive forces to a larger extent.
A branched hydrocarbon generally has a smaller surface area than its corresponding straight-chain isomer, and therefore, branching causes a decrease in boiling point.
Comparing boiling point of compounds
Are there any dipole-dipole interactions in either compound?
Identify all dipole-dipole interactions in both compounds.
Will either compound form hydrogen bonds?
How many carbon atoms are in each compound?
How much branching is in each compound?
Proton transfer curved arrow notation
STEP 1 Identify the acid and the base
STEP 2 Draw the first curved arrow. The tail of one curved arrow is placed on a lone pair of the base, and the head is placed on the proton of the acid. This first curved arrow shows the base abstracting the proton. The next curved arrow always comes from the bond being broken and goes to the atom connected to the proton: STEP 3 Draw the second curved arrow.
Ka
The value of Ka measures the strength of the acid. Very strong acids can have a Ka on the order of 1010 (or 10,000,000,000), while very weak acids can have a Ka on the order of 10−50
pKa
A strong acid will have a low pKa value, while a weak acid will have a high pKa value.
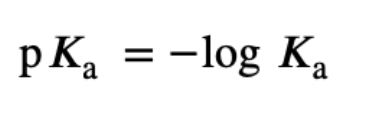
Using pKa to compare basicity
STEP 1 Draw the conjugate acid of each base. We just need to compare the pKa values of those acids.
STEP 2 Compare pKa values.
STEP 3 Identify the stronger base. Remember that the stronger acid always generates the weaker base.
Assessing stability of anions
We simply look at their conjugate bases, A− and B−, and compare them to each other:
By determining the more stable conjugate base, we can identify the stronger acid.
A: ATOM: there are two important trends: electronegativity (for comparing atoms in the same row) and size (for comparing atoms in the same column).
The second factor for comparing conjugate base stability is resonance.
Sharing electrons over more atoms is better
I: INDUCTION
The net effect of the EN atoms is to withdraw electron density away from the negatively charged region of the structure, thereby stabilizing the negative charge.
O: ORBITAL
The answer here comes from looking at the hybridization states of the orbitals that accommodate the charges.
A pair of electrons in an sp-hybridized orbital is held closer to the nucleus than a pair of electrons in an sp2- or sp3-hybridized orbital. As a result, electrons residing in an sp orbital are stabilized by being close to the positively charged nucleus.
ARIO
Atom. Which atom bears the charge? (How do the atoms compare in terms of electronegativity and size? Remember the difference between comparing atoms in the same row vs. atoms in the same column.)
Resonance. Are there any resonance effects that make one conjugate base more stable than the other?
Induction. Are there any inductive effects that stabilize one of the conjugate bases?
Orbital. In what orbital do we find the negative charge for each conjugate base?
Assessing stability of cationic acids (+ charged)
In contrast, to assess the acidity of cationic acids (HA+ vs. HB+), it is often not necessary to compare their conjugate bases.
Rather, we can compare the stability of the positive charges in the cationic acids (HA+ and HB+).
ARIO for charged vs uncharged acids
For uncharged acids (HA vs. HB), we focus on the stability of the negative charges in the conjugate bases; and for cationic acids (HA+ vs. HB+), we can focus on the stability of the positive charges in the acids themselves.
Predicting the position of equilibrium with pKa
STEP 1 Identify the ion on either side of the equilibrium.
STEP 2 Compare the stability of these two ions.
STEP 3 The equilibrium will favor the more stable ion.
Equilibrium favors… (in terms of charge)
The equilibrium will favor the more stabilized anion. If A− is more stable, then the equilibrium will favor the formation of A−. If B− is more stable, then the equilibrium will favor the formation of B−. So the position of equilibrium can generally be predicted by comparing the stability of A− and B−.
In this type of acid-base reaction, the equilibrium will favor the more stabilized cation. If HA+ is more stable, then the equilibrium will favor the formation of HA+. If HB+ is more stable, then the equilibrium will favor the formation of HB+. So the position of equilibrium can generally be predicted by comparing the stability of HA+ and HB+.
Choosing an appropriate reagent
STEP 1 Draw the equilibrium.
STEP 2 Compare the stability of the bases on either side of the equilibrium using all four factors.
STEP 3 The reagent is only suitable if the equilibrium favors the desired products.
If products are favored, the reaction is useful.
If starting materials are favored, the reaction is not useful.
Leveling effect
If a base stronger than HO− is dissolved in water, the base reacts with water to produce hydroxide.
This is called the leveling effect, and it dictates the strongest base that can be present in any particular solvent.
Specifically, the base cannot be stronger than the conjugate base of the solvent. If the solvent is water, than hydroxide is the strongest base that can be used.
In a basic solution, the base cannot be stronger (less stable) than the conjugate base of the solvent; and in an acidic solution, the acid cannot be stronger than the conjugate acid of the solvent.
Solvating effects
The more sterically hindered molecule will be less capable of interacting with the solvent
The less “bulky” or branched the molecule is, the better it solvates
Lewis acid and base
A Lewis base is defined as an electron-pair donor, while a Lewis acid is defined as an electron-pair acceptor.
Identifying Lewis acids and bases
STEP 1 Identify the direction of the flow of electrons. Which reagent is serving as the electron-pair donor and which reagent is serving as the electron-pair acceptor?
STEP 2 Identify the electron-pair acceptor as the Lewis acid and the electron-pair donor as the Lewis base
Bronsted-Lowry acid and base
The definition of Brønsted-Lowry acids and bases is based on the transfer of a proton (H+). An acid is defined as a proton donor, while a base is defined as a proton acceptor.
Drawing bond line structures
Carbon atoms in a straight chain should be drawn in a zigzag format
When drawing double bonds, draw all bonds as far apart as possible:
When drawing single bonds, the direction in which the bonds are drawn is irrelevant
All heteroatoms (atoms other than carbon and hydrogen) must be drawn, and any hydrogen atoms attached to a heteroatom must also be drawn.
Oxygen formal charge patterns
A negative charge corresponds with one bond and three lone pairs.
The absence of charge corresponds with two bonds and two lone pairs.
A positive charge corresponds with three bonds and one lone pair.
Nitrogen formal charge patterns
A negative charge corresponds with two bonds and two lone pairs.
The absence of charge corresponds with three bonds and one lone pair.
A positive charge corresponds with four bonds and no lone pairs.
Carbon formal charge patterns
In summary, both C+ and C− will have only three bonds. The difference between them is the nature of the fourth orbital. In the case of C+, the fourth orbital is empty. In the case of C−, the fourth orbital is occupied by a lone pair of electrons.
wedge and dash notation
a wedge represents a group coming out of the page, and a dash represents a group going behind the page
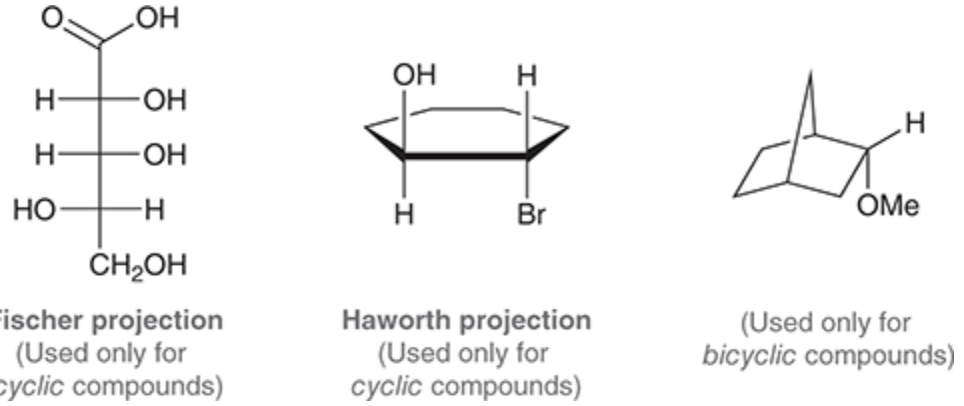
Fisher and Haworth projections
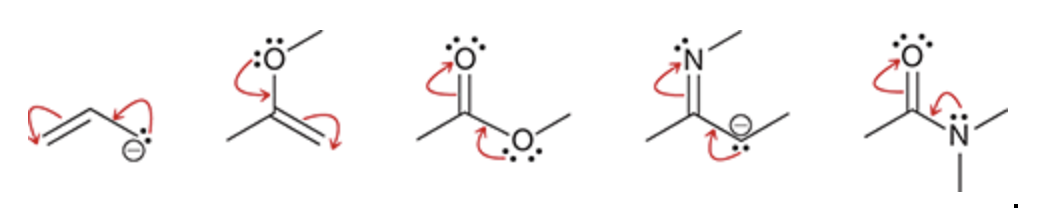
Curved arrow rules for resonance
Avoid breaking a single bond.
Never exceed an octet for second-row elements.
Allylic lone pair
When a compound contains a carbon-carbon double bond, the two carbon atoms bearing the double bond are called vinylic positions, while the atoms connected directly to the vinylic positions are called allylic positions:
The first curved arrow goes from the lone pair to form a π bond, while the second curved arrow goes from the π bond to form a lone pair:
When the atom with the lone pair has a negative charge, then it transfers its negative charge to the atom that ultimately receives a lone pair:
When the atom with the lone pair does not have a negative charge, then it will incur a positive charge, while the atom receiving the lone pair will incur a negative charge:
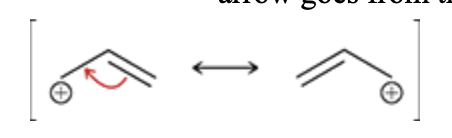
Allylic carbocation
Again we are focusing on allylic positions, but this time, we are looking for a positive charge located in an allylic position:
When there is an allylic carbocation, only one curved arrow will be required; this arrow goes from the π bond to form a new π bond:
Never place the tail of a curved arrow on a positive charge (that is a common mistake).
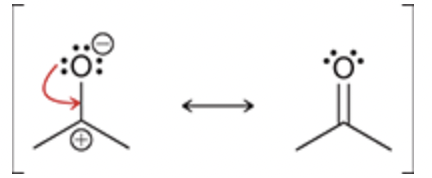
Lone pair adjacent to positive carbon
This pattern requires only one curved arrow. The tail of the curved arrow is placed on a lone pair, and the head of the arrow is placed to form a π bond between the lone pair and the positive charge:
The atom with the lone pair has a negative charge in this case, and therefore the charges end up canceling each other.
Pi bond between two atoms with differing EN
In these situations, we move the π bond up onto the electronegative atom to become a lone pair:
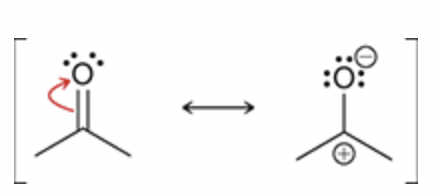
A double bond is being separated into a positive and negative charge
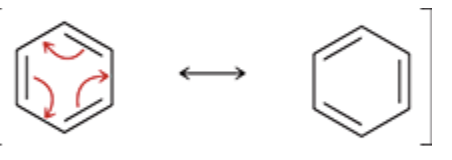
Conjugated π bonds enclosed in a ring.
Earlier in this section, we referred to π bonds as being conjugated when they are separated from each other by one σ bond
When conjugated π bonds are enclosed in a ring of alternating double and single bonds, we can push all of the π bonds over by one position:
Choosing the most significant resonance structure
The most significant resonance forms have the greatest number of filled octets.
It is fairly common to encounter a carbon atom with a positive charge, even though it lacks an octet (as seen in the first resonance form above). In contrast, oxygen is much more electronegative than carbon, so you should never draw a resonance form in which an oxygen atom lacks an octet.
The structure with fewer formal charges is more significant.
Any resonance form that contains an atom bearing a +2 or −2 charge is highly unlikely.
Other things being equal, a structure with a negative charge on the more electronegative element will be more significant.
Similarly, a positive charge will be more stable on the less electronegative element.
Drawing a resonance hybrid
STEP 1 Draw the significant resonance structures.
STEP 2 Determine the relative significance of each resonance structure.
STEP 3 Draw the average of the resonance structures to indicate partial bonds and partial charges.
STEP 4 Refine the drawing by drawing the partial charges to reflect the relative significance of the individual resonance structures, as appropriate.
The more significant resonance structure will contribute more character to the resonance hybrid.
The negative charge is still shared between the oxygen and carbon atoms; however, more of the negative charge will reside on the more electronegative oxygen atom. This unequal sharing is indicated by using differently sized δ− signs, highlighted in the following revised resonance hybrid.
Resonance orbital occupied by lone pair
When an atom possesses a delocalized lone pair, the geometry of that atom is affected by the presence of the lone pair.
Whenever a lone pair participates in resonance, it will occupy a p orbital rather than a hybridized orbital, and this must be taken into account when predicting geometry.
A localized lone pair, by definition, is a lone pair that does not participate in resonance. In other words, the lone pair is not allylic to a π bond
Whenever an atom possesses both a π bond and a lone pair, they will not both participate in resonance. In general, only the π bond will participate in resonance, and the lone pair will not.
one
meth
two
eth
three
prop
four
but
five
pent
six
hex
seven
hept
eight
oct
nine
non
ten
dec
eleven
undec
twelve
dodec
thirteen
tridec
fourteen
tetradec
fifteen
pentadec
20
eicos
30
triacont
40
tetracont
50
pentacont
100
hect
Alkane nomenclature process
Identify the parent chain (the longest consecutive chain of carbons)
2. Identify and Name the substituents
Count the number of
carbons in each side group
• Use yl instead of ane
3. Number the parent chain and assign a locant (and prefix if necessary) to each
substituent
Give the first substituent the lowest number possible
4. List the numbered substituents before the parent name in alphabetical order
Ignore prefixes (except iso) when ordering alphabetically
size of alkanes
At room temperature
– Small alkanes with 1-4 carbons are gasses
– Medium size alkanes with 5-12 carbons are liquids
– Large alkanes with 13-20 carbons are oils
– Extra large alkanes with 20-100 carbons are solids like tar and wax
– Super-sized alkanes called polymers can have thousands or millions of
carbon atoms in each molecule
Rings in nomenclature
If the parent chain is cyclic (a ring of carbons), add the prefix, “cyclo” to the
beginning of the parent name
A ring can be either a parent chain or a substituent depending on the number of
carbons
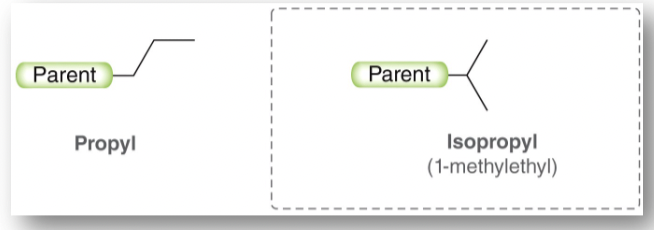
Isopropyl
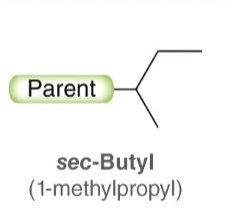
sec-Butyl
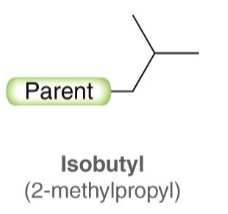
Isobutyl
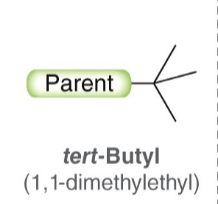
tert-Butyl
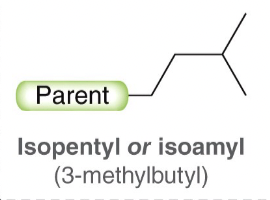
Isopentyl or isoamyl
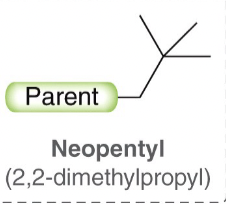
Neopentyl
Curved arrows in acid/base
The making and breaking of bonds involves electron movement.
• We use curved arrows to describe the flow of electron density.
• The are the same as curved arrows used to draw resonance structures,
but...here, the curved arrows are actually describing the physical
movement of electrons!!!
Equilibrium favors…
weaker acid and weaker base
Determining relative acidity
To determine the relative strength of two acids, without knowing their pK a
values, we compare the stability of their conjugate bases.
• The stronger the acid, the more stable its conjugate base!
• When an acid loses a proton, it forms the conjugate base, which has a lone
pair of electrons that resulted from the loss of H + .
To determine the stability of a conjugate base, we are actually looking at the
stability of the lone pair.
Predicting eqilibrium
There are two distinct ways to predict which side is favored at equilibrium.
1. The pK a values of H—A and H—B (the higher pKa will be favored).
2. The relative stability of the bases, B- and A -.
If pK a values are not known, relative stability of conjugates should be used
pKa of water
15.7