Reservoir Thermodynamics KNW
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
58 Terms
hydrocarbon reservoir
an accumulation of hydrocarbon (in gas, liquid, solid or combo state) in porous permeable sedimentary or fractured rock formations.
HC at STP formula
STHIIP = Vø(1-Swi)/BHi
V = gross/ bulk rock volume
ø = porosity
Swi = initial water saturation
BHi = initial HC formation volume
STP
T = 15°C = 60°F
P = 101325 Pa = 14.7 psia
principles of EOR
improve mobility ratio
increase capillary number
Why do we need to know about reservoir fluids?
multiphase flow
suitability for surface facilities
environmental & health issues
compatibility
What do we need to know about reservoir fluids?
composition
solution gas oil ratio, Rs
formation volume factor, Bo Bg
density
viscosity
IFT, surface tension
PT
compositional analysis
the measurement of the distribution of HC and other components present in oil and gas samples using modern chromatography techniques
HC grouping criteria
molecular configuration: straight chin, branched, cyclic or aromatic
number of carbon bonds: single, double & triple
HC groups
aliphatic compounds: alkanes (CnH2n+2), alkenes (CnH2n), alkynes (CnH2n-2)
saturated → single bonds
unsaturated → at least one double/ triple bond
aromatic compounds: arenes
contain unsaturated cyclic compounds
react readily bc of C=C bond
formula: C6H6(CH2)n
compounds present in the reservoir
HC
inorganic compounds: He, N2, CO2, H2S, water
sulfur/ sour compounds: H2S, RSH, RSR, RSSR
non-HC elements: Hg, Ni, V, radioactive elements
solid like compounds: waxes, resins, asphaltenes, diamondoids, hydrates
intensive property
a property which is independent of the system mass (quantity) = bulk property e.g.
activity/ fugacity
them potential
density/ hardness/ roughness
specific/ molar enthalpy/ entropy/ Helmholtz free energy/ internal energy/ gibs free energy/ heat capacity/ volume
isothermal compressibility
pT
thermal conductivity/ diffusivity
volumetric thermal expansion
extensive property
a property which is dependent of the system mass (quantity) = additive property e.g.
Helmholtz free energy
enthalpy
internal energy
entropy
gibs free energy
heat capacity
mass
volume/ length
charge
thermodynamic state
the macroscopic condition of a thermodynamic system at a particular time determined when all intensive properties are fixed.
steady → a system has numerous properties that are unchanging in time (time-invariant or equilibrium), but the system is either open or closed.
unsteady → a process variable (property) has been changed, and the system has not yet reached a steady state (i.e., local equilibrium or partial equilibrium).
homogeneous system
a thermodynamic system whose intensive properties change continuously and uniformly (smoothly). i.e., chemical composition and physical properties are the same in all parts of the system or change continuously from one point to another
heterogeneous systems
a thermodynamic system consisting of two or more homogeneous bodies (phases). Each phase is separated from other phases by interfaces or boundaries, and in passing over such a boundary, the chemical composition of the substance or its physical properties abruptly changes.
phase
a restricted part of a system with distinct physical and chemical properties.
state functions/ variables
a system property that depends only on the system’s current (equilibrium) state → allow quantifying any change of a system e.g. enthalpy, entropy & internal energy
path function
a system property that depends on the specific transition (or path) between two equilibrium states. e.g. mechanical work & heat
process
any change that a system undergoes from one equilibrium state to another.
reversible → a process that can be reversed without leaving any trace on the surroundings
carried out infinitesimally slowly
idealisation of processes
irreversible → a process in that change is brought about rapidly, and the system does not attain equilibrium.
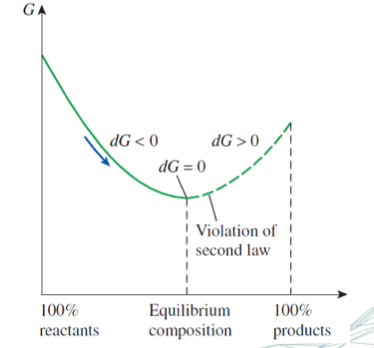
equilibrium = state of rest
no further change or - more precisely - no net-flux will take place unless one or more properties of the system are altered.
(dG)T,p = 0
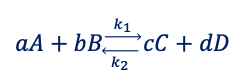
concentration
the amount of solute in a solution mixture
mass concentration
the mass of a solute dissolved in a unit volume of a solution
𝜌solute = msolute/ Vsolution [kg/m3]
molar concentration = molarity
the number of moles of a solute dissolved in a unit volume of a solution
M = csolute = nsolute/ Vsolution [mol/lit]
1 mol/lit = 1000 mol/m3
volume concentration = volume fraction
the volume of a solute dissolved in a unit volume of a solution
øsolute = Vsolute/ Vsolution [m3/m3]
molality
the number of moles of a solute dissolved in a unit mass of a solvent
m = nsolute/ msolvent [mol/kg of volvent]
internal energy, U
energy of a system, i.e., the energy associated with the random motion of the molecules within a system = Associated with the temperature of the system.
U = Q + W [J]
kinetic = translational, rotational & vibrational
potential = static energy of atoms & chemical bonds
1.law of thermodynamics
change in the total macroscopic energy of a system is equal to the difference between the total amount of heat supplied to the system and the amount of work done by the system on its surroundings.
dE = 𝛿Q - 𝛿W
d(Ekin) + d(Epot) + dU = 𝛿Q - 𝛿W
dU = 𝛿Q - 𝛿W
∆U = Q - W
enthalpy, H
a measure of the total heat content of a thermodynamic system at constant pressure conditions.
H = U + pV [J]
∆H = ∆U + ∆(pV)
∆H = Q - W + ∆(pV). & W = p∆V => ∆H = Q at KE, PE = 0
entropy, S
the measure of the tendency to change and the direction in which change can occur = Potential of an macroscopic system to be described by different microscopic states.
dS ≥ 𝛿Q/T [J/K]
2.law of thermodynamics
the entropy of the universe increases in a spontaneous process and remains unchanged in an equilibrium process.
∆𝑆universe> 0 spontaneous process
∆𝑆universe= 0 reversible (equilibrium) process
∆𝑆universe= ∆𝑆system + ∆𝑆surrondings
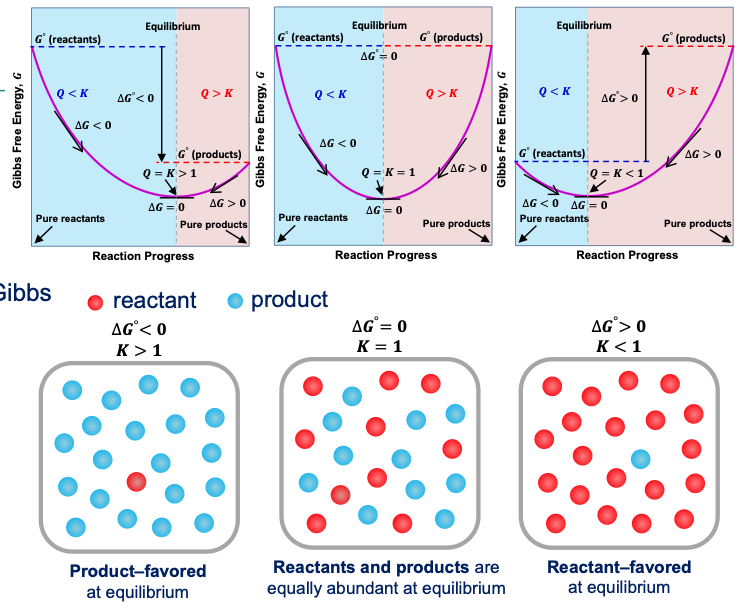
Gibbs free energy, G
a thermodynamic property that predicts whether a process will occur spontaneously at constant temperature and pressure. → reactions move in the direction of decreasing dG
𝐺= 𝐻 − 𝑇𝑆 [J]
∆𝐺 < 0 spontaneous processes
∆𝐺 > 0 nonspontaneous processes
Helmholtz free energy, A
a thermodynamic property that predicts whether a process will occur spontaneously at constant temperature and volume.
A = U - TS [J]
∆𝐴 < 0 spontaneous processes
∆𝐴 > 0 nonspontaneous processes
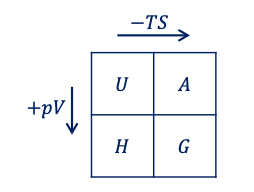
thermodynamic processes
adiabatic → adiabatic (no heat added to or removed from the system),
isothermal → constant temperature
isobaric → constant pressure
isochoric → constant volume
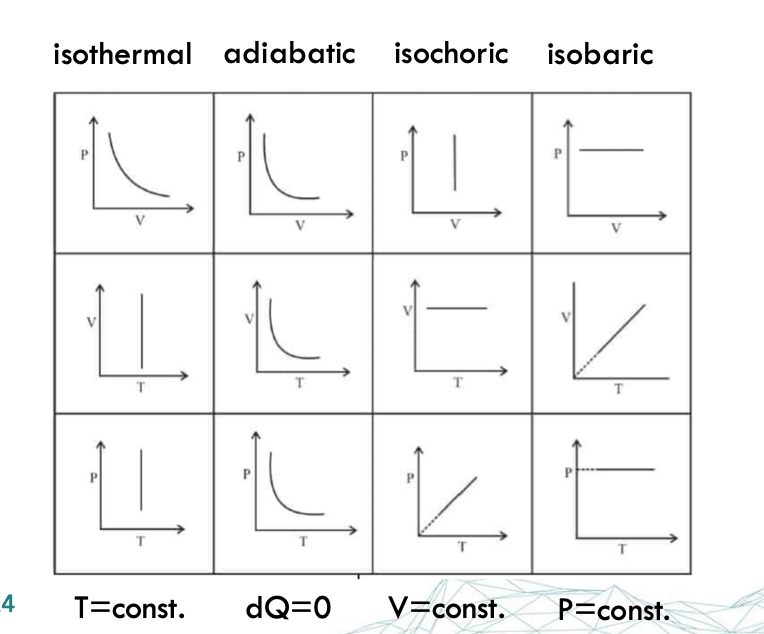
thermodynamic systems
a portion of the universe defined by appropriate boundaries (physical or virtual)
open → allowing exchange of mass, heat, and work energy with the surroundings
closed → allowing exchange of heat or work energy with the surroundings but not mass
isolated → allowing neither exchange of mass nor energy in any form with the surroundings
adiabatic/ insulated → allowing exchange of mass and work energy with the surroundings but not heat
sour gas
any gas that specifically contains hydrogen sulfide in significant amounts (>5.7 mg of H2S per cubic meter of natural gas).
acid gas
any gas that contains significant amounts of acidic gases such as carbon dioxide (CO2) or hydrogen sulfide.
natural gas composition
C1-7 (HC) + CO2/H2S/N2 (non HC)
component
a chemically independent constituent of a system
→ the minimum number of such chemical units or species (ions/molecules) required to describe the composition of all the phases present in the system.
gas viscosity characteristics
directly proportional
at low p = increases as T increases
at high p = decreases as T increases
general oil viscosity characteristics
decrease w/ increasing T
increase w/ increasing p
saturated oil viscosity characteristics
decreases w/ increasing p due to the increasing fraction of dissolved light components
decreases w/ increasing T
typical oil viscosities
crude: 0.5 -10 cP
heavy oils: » 100 cP
IFT properties
decreases w/ increasing T
effected by pH
typical range: 20-150 mN/m
IFT influence
capillary pressure
residual saturation
shape of relative permeability curves
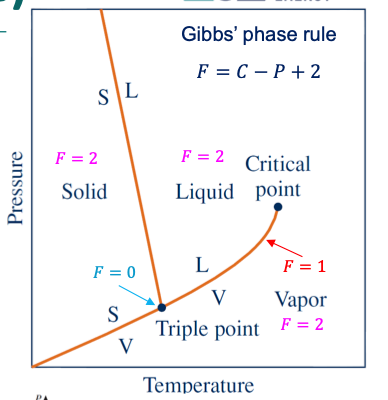
degree of freedom, F
the number of variables that may be varied independently without changing the number of phases present at equilibrium.
F = 0 → invariant: change the no. of present phases (points)
F = 1 → univariat: the value of one property may be adjusted without changing the number of phases (lines)
F = 2: bivariant: the values of two properties may be adjusted independently without a change in the number of phases present (areas)
Gibbs’ phase rules
determine the variance (degrees of freedom) of any system at equilibrium.
𝐹 = 𝐶 − 𝑃 + 2
If the substance is present as only one phase, e.g., a solid, then P = 1
If the system exists as two phases in equilibrium (e.g., vapor and liquid), then P = 2
If the system exists as three phases in equilibrium (solid, liquid, and vapor), then P = 3
vapour quality
the ratio of the mass of vapor to the total mass of the saturated mixture
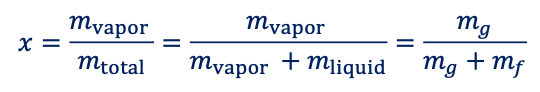
Characteristics of Two Component Systems
pcrit > pcrit, pure comp
Tcrit between Tcrit pure components
BPtemp > BPtemp, pure light
DPtemp < DPtemp, pure heavy
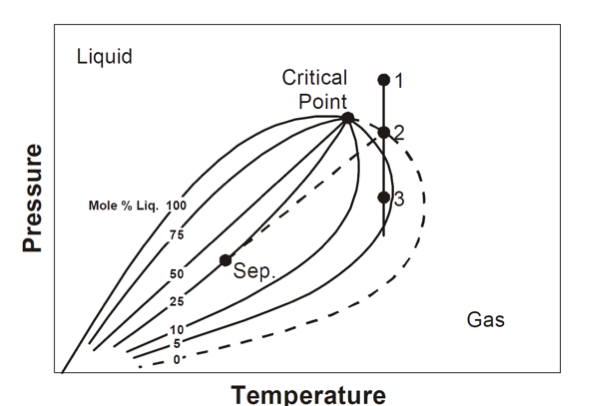
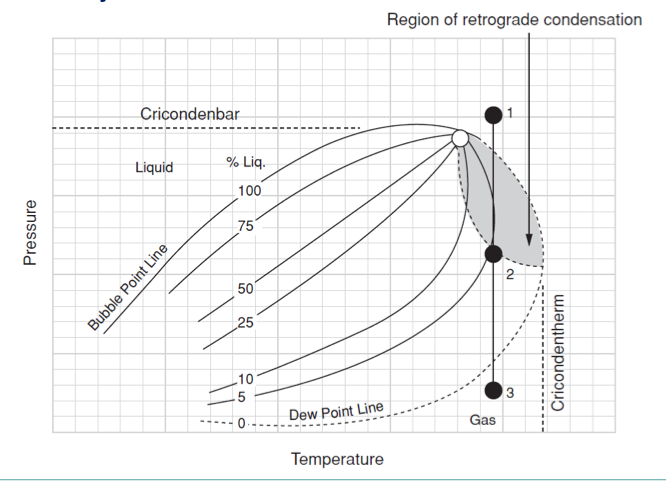
phase envelope diagram components
BP curve
DP curve
critical point
quality lines
cricondentherm (Tct)
cricondenbar (Pcb)
reservoir fluids classification
large to small molecules
black oil
volatile oil
gas condensate
wet gas
dry gas
reservoir fluids classification parameters
initial producing gas oil ratio, GOR
gravity of the stock tank liquid, API
composition
black oil reservoir
GORi < 2000 [scf/stb] → increases during production, below pb
15 < °API < 40
brown to dark green
low vs high shrinkage oil
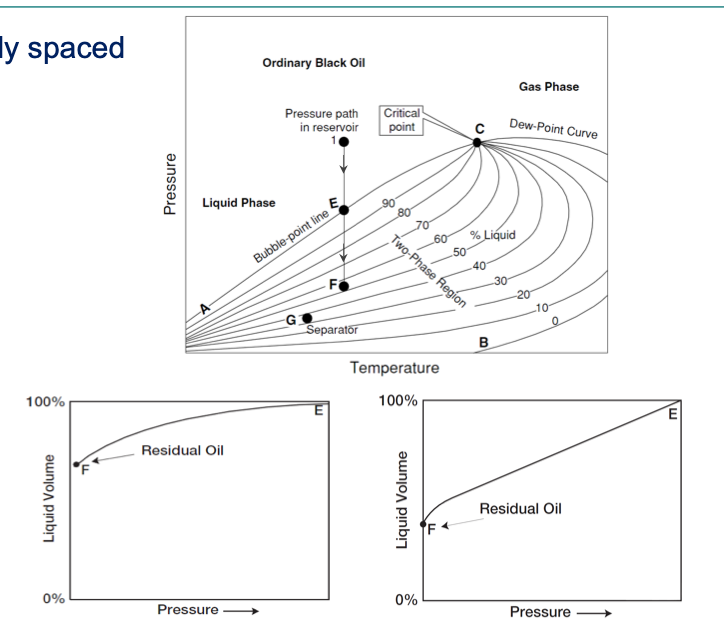
volatile oil reservoir
2000 < GORi < 3300 [scf/stb]→ increases during production
40 < °API < 50
Larger fraction of light and intermediate components
Greenish to orange
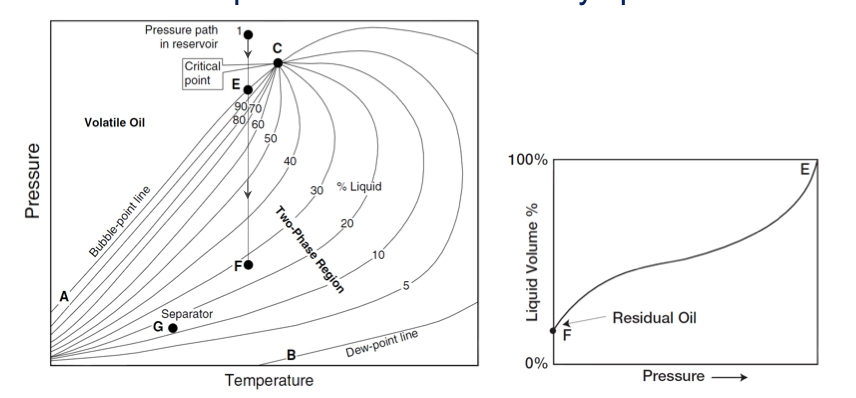
gas condensate reservoir
Tc < Tres < Tct
3300 < GORi < 50000 [scf/stb]
50 < °API < 70
Translucent or slightly colored
dry gas reservoir
The hydrocarbon mixture exists as a gas both in the reservoir and in the surface
Tres > Tct
GORi > 100000 [scf/stb] → no surface liquids
C1 and smaller fraction of intermediates components (C2-C6) and non-HC components (N2, CO2, etc.)
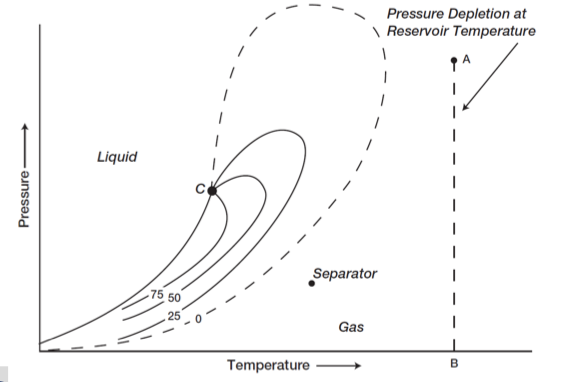
wet gas reservoir
The hydrocarbon mixture exists as a gas in the reservoir and two-phase in the surface
Tres > Tct
50000 < GORi < 100000 [scf/stb] → will remain constant
60 < °API < 70
C1 and larger fraction of intermediate components (C2- C6)
translucent
retrograde condensation
the pressure is reduced below the dew point, the volume of liquid in the two-phase mixture initially increases
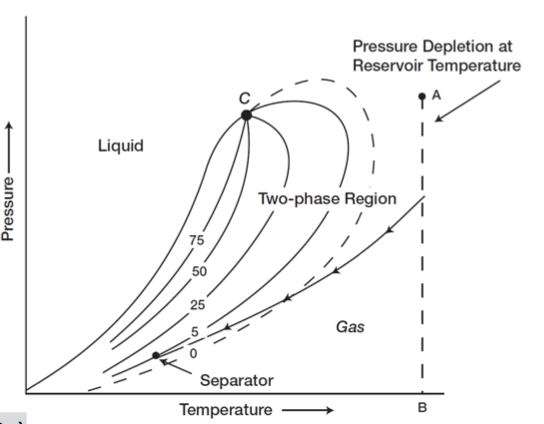
condensate banking
When liquid condensate builds up (or 'banks') near the wellbore or in low spots in a pipeline.
This can happen in gas condensate reservoirs when the pressure drops below a certain level (called the dew point), causing the gas to turn into liquid.