3.1 -3.3 ENZYMES
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
What is an Enzyme
An enzyme is a globular protein that acts as a biological catalyst by speeding up the rate of a chemical reaction.
Enzymes are not changed or consumed by the reactions they catalyze and thus can be reused.
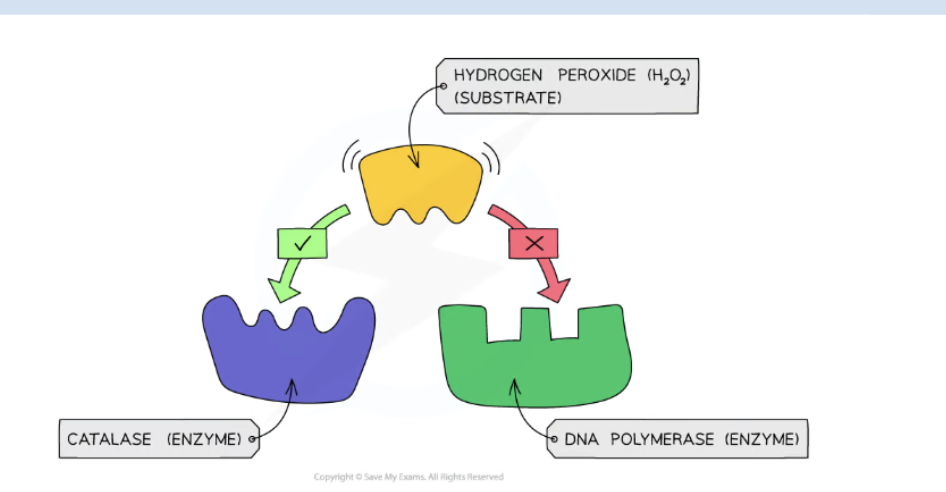
Enzymes are typically named after the molecules they react with (called the substrate) and end with the suffix “-ase”. Example – lipids are broken down by the enzyme lipase
Active Site
The active site is the region on the surface of the enzyme which binds to the substrate molecule.
The active site and the substrate complement each other in terms of both shape and chemical properties. Hence only a specific substrate is capable of binding to a particular enzymes’s active site.
For an enzyme-mediated chemical reaction to occur, the shape and charge of the substrate must be compatible with the active site of the enzyme.
When enzyme and substrate bind together they for an enzyme-substrate complex
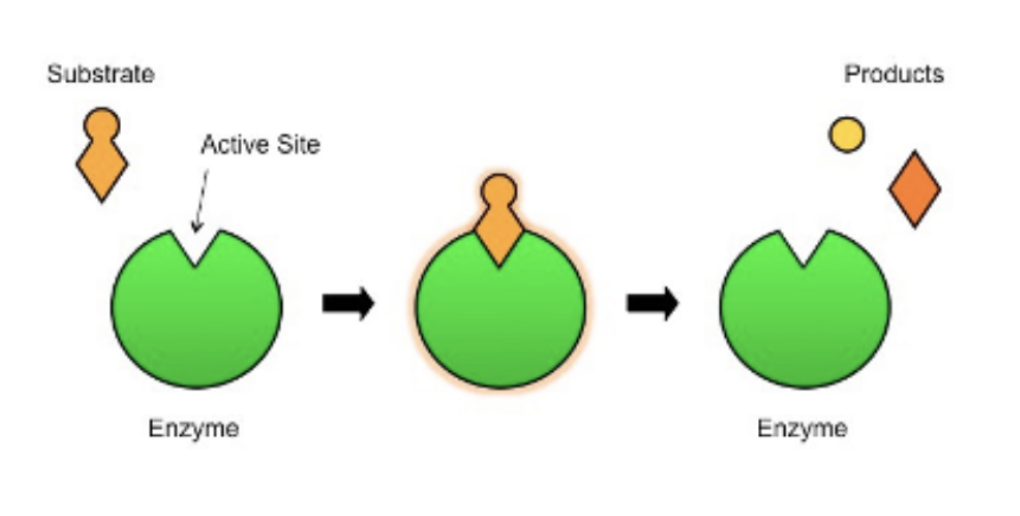
Enzyme Catalysis
The substrate needs to be brought into physical proximity to the enzyme for substrate enzyme interaction.
1, an enzyme has a cleft in its substrate, called the active site. The substrate molecule has a complementary shape
2, Random movement of enzyme and
substrate brings the substrate into the
active site. An enzyme-substrate
complex is temporarily formed. The
R groups of the amino acids in the
active site interact with the substrate.
3, The interaction of the substrate with the
active site breaks the substrate apart.
An enzyme-product complex is briefly
formed, before the two product
molecules leave the active site, leaving
the enzyme molecule unchanged and
ready to bind with another
substrate molecule.
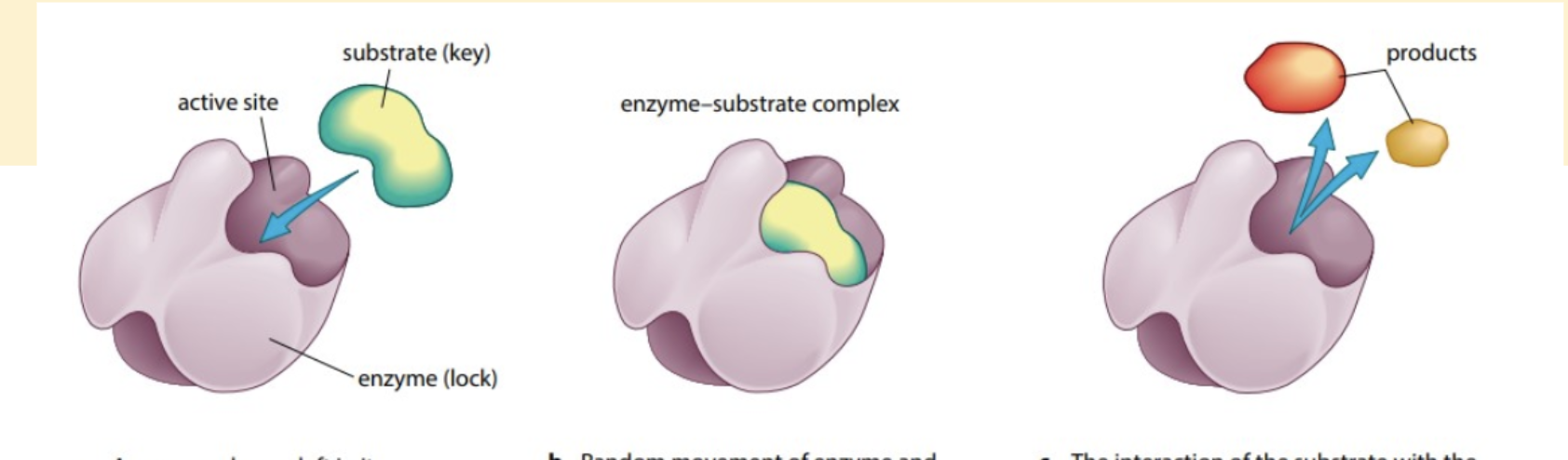
Induced fit model
It explains how enzymes may exhibit broad specificity (e.g. lipase can bind to a variety of lipids)
It explains how catalysis may occur (the conformational change stresses bonds in the substrate, increasing reactivity)
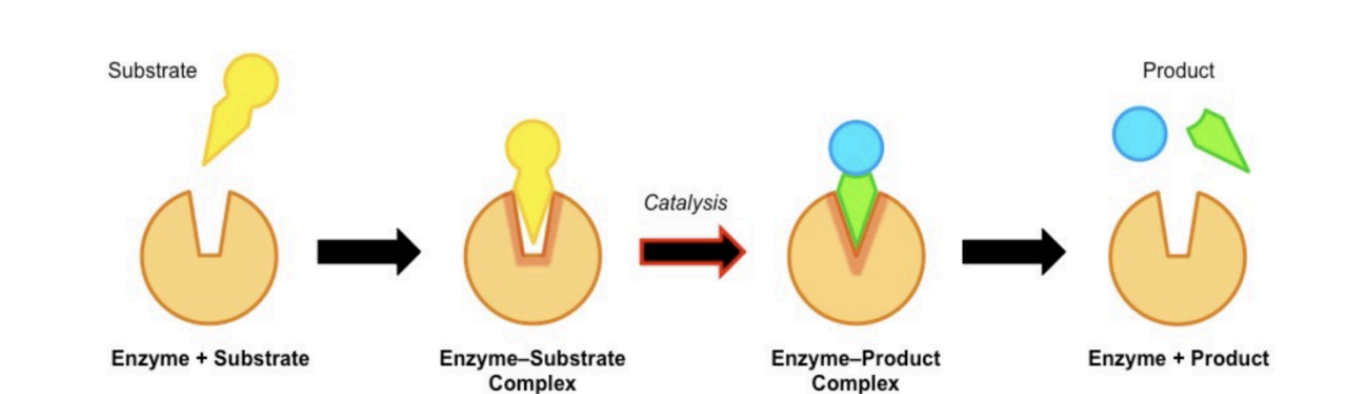
Enzyme substrate specificity
All enzymes possess an active site which complements the substrate. This active site is responsible for enzyme-substrate specificity.
The shape and chemical properties of the active site are highly dependent on the tertiary structure of the enzyme protein.
Active site and properties of an enzyme
The structure of enzymes includes the active site that specifically interacts with substrate molecules.
For an enzyme-mediated chemical reaction to occur, the shape and charge of the substrate must be compatible with the active site of the enzyme
Collision Frequency
The rate of enzyme catalysis can be increased by improving the frequency of collisions via:
● Increasing the molecular motion of the particles (thermal energy can be introduced to increase kinetic energy)
● Increasing the concentration of particles (either substrate or enzyme concentrations)
Higher environmental temperatures increase the speed of movement of molecules in a solution, increasing the frequency of collisions between enzymes and substrates and therefore increasing the rate of reaction.
Effect of temperature and pH on enzyme
Like all proteins, enzyme structure can be modified by external factors such as high wtemperatures and extreme pH
● These factors disrupt the chemical bonds which are necessary to maintain the tertiary
structure of the enzyme.
● Any change to the structure of the active site (denaturation) will negatively affect the
enzyme’s capacity to bind the substrate.
protein denaturation
Protein denaturation is a process where a protein loses its natural shape and structure.
This can happen due to changes in temperature, pH levels, or exposure to chemicals.
When a protein denatures, it unfolds and loses its functional shape, which can affect its ability to perform its normal biological functions.
Effect of temperature on enzyme activity
Low temperature results in insufficient thermal energy for the activation of enzyme catalysed reaction to proceed.
Increasing temperature will increase the speed and motion of both enzyme and substrate, resulting in higher enzyme activity.
This is because a higher kinetic energy will result in more frequent and strong collisions between enzymes and substrates.
an optimum temperature the rate of enzyme activity will be maximum.
Higher temperature (beyond the optimum temperature) will affect enzyme stability. Thermal energy will disrupt hydrogen bonds of the enzyme and enzyme will lose its shape (the active site loses shape) resulting in loss of activity (denaturation)

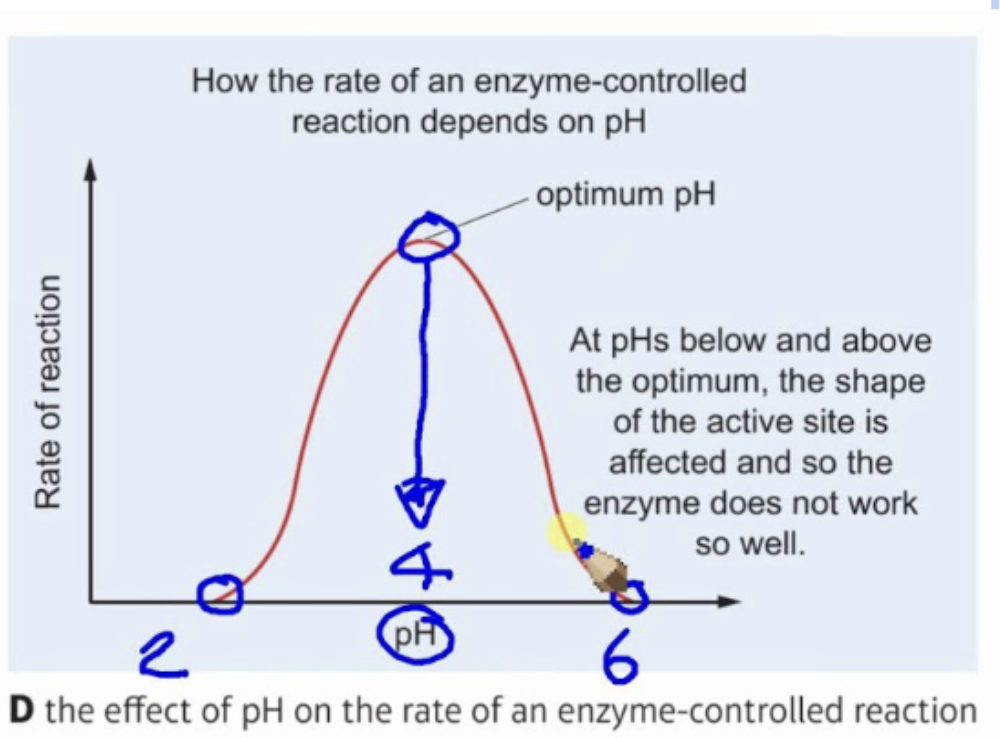
Effect of pH on enzyme activity
Changing pH will alter the charge of the enzyme, which in turn will alter the protein structure and shape.
changing the pH to an extreme will disrupt the hydrogen bonds and change the shape of active site.
Change in shape of active site and lower its ability to bind the substrate making enzyme lose its function (denature)
enzymes have optimum pH and moving outside this range lowers its activity.
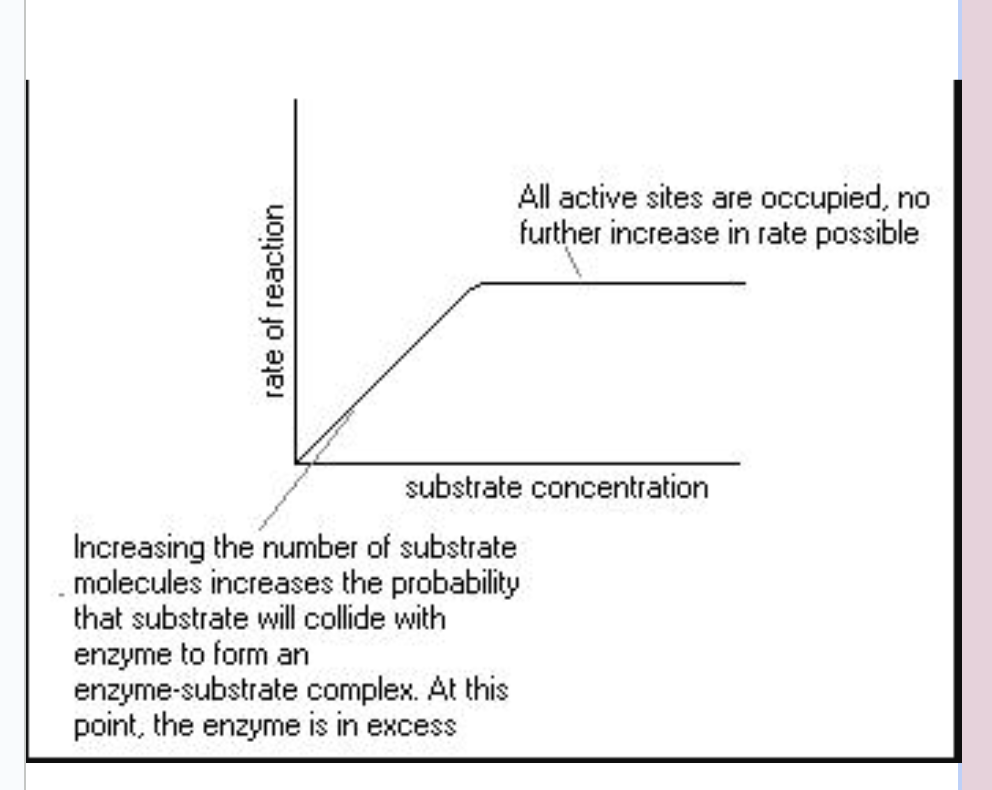
Effect of substrate concentration on enzyme activity
increasing substrate concentration will increase the activity of a corresponding enzyme.
more substrate molecules means there is an increased chance of enzyme and substrate colliding and reacting within a given period.
After a certain point, the activity will cease to rise regardless of any further increase in substrate concentration.
This is because the environment is saturated with substrate and all enzyme active sites are bound with substrate.
effect of enzyme concentration on enzyme activity
As the amount of enzyme increase, the rate of reaction increase. If there are more enzyme molecules than are needed, adding additional enzyme molecules will not increase the rate of reaction as availability of substrate becomes a limiting factor.
Effect of product concentration on enzyme activity
The enzyme activity declines when the products start accumulating. This is called Product or Feedback inhibition.
For certain enzymes, the product combines with the active site of enzyme and forms a loose complex and thus inhibits the enzyme activity.
Explain how the cellular environment affects enzyme activity.
Change to the molecular structure of a component in an enzymatic system may result in a change of the function or efficiency of the system—
a. Denaturation of an enzyme occurs when the protein structure is disrupted, eliminating the ability to catalyze reactions.
b. Environmental temperatures and pH outside the optimal range for a given enzyme will cause changes to its structure, altering the
efficiency with which it catalyzes reactions.
enviornmental impacts on enzyme action
Environmental pH can alter the efficiency of enzyme activity, including through disruption of hydrogen bonds that provide enzyme structure.
he relative concentrations of substrates and products determine how efficiently an enzymatic reaction proceeds.
Cellular Energy
Activation energy is the energy required to initiate a reaction
Enzymes lower the activation energy, allowing reactions to occur more readily.
Lowering the activation energy of a reaction by enzymes does not affect the energy of the products or the reactants in the chemical reaction. Instead, it reduces the energy barrier that must be overcome for the reactants to transition into the products. This does not affect the energy of the products formed
Explain how enzymes affect the rate of biological reactions.
The structure and function of enzymes contribute to the regulation of biological processes—
Enzymes are biological catalysts that facilitate chemical reactions in cells by lowering the activation energy.
Enzyme Inhibition
An enzyme inhibitor is a molecule that disrupts the normal reaction pathway between an enzyme and a substrate.
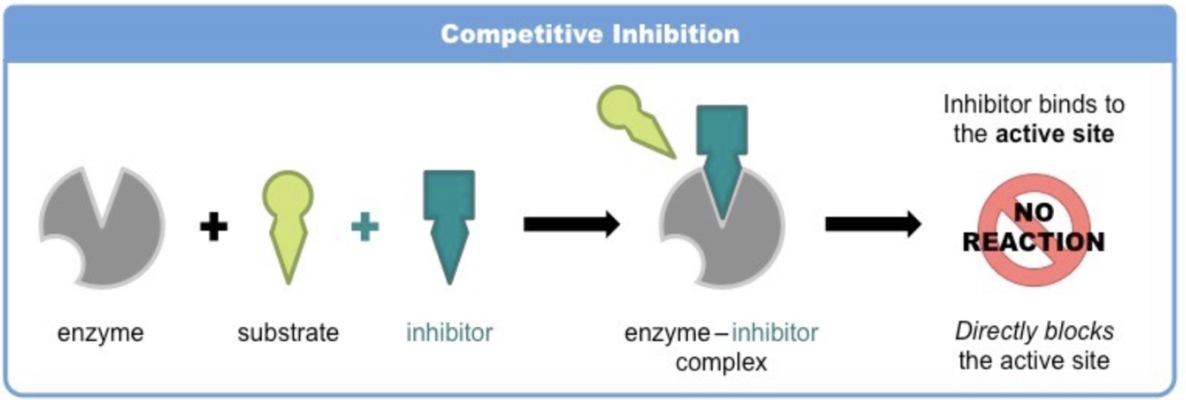
Competitive inhibition
The molecule (inhibitor) is structurally and chemically similar to the substrate (hence able to bind to the active site).
As the inhibitor is in competition with the substrate, its effects can be reduced by increasing substrate concentration
Non Competitive Inhibition
Non-competitive inhibition involves a molecule binding to a site other than the active site (an allosteric site)
The binding of the inhibitor to the allosteric site causes a conformational change to the enzyme’s active site
Enzyme inhibition
Competitive inhibitor molecules can bind reversibly or irreversibly to the active site of the enzyme.
Noncompetitive inhibitors can bind to allosteric sites, changing the activity of the enzyme.