Comprehensive AKI, CKD, Osteoarthritis, and Osteoporosis Pharmacology and Pathophysiology
1/108
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
109 Terms
What is Acute Kidney Injury (AKI)?
A sudden decrease in kidney function over hours or days, also known as acute renal failure.
What are the main manifestations of AKI?
Decreased urine output (oliguria), acute increase in serum creatinine and blood urea nitrogen.
What can result from AKI?
Disturbance of extracellular fluid volume, electrolyte imbalance, acid-base abnormalities, and retention of nitrogenous waste products.
Is AKI reversible?
Yes, if detected early and treated appropriately.
What are the three classifications of AKI causes?
Pre-renal, renal (intrinsic), and post-renal.
What is a pre-renal cause of AKI?
A decrease in renal blood flow or perfusion to nephrons.
What are some examples of pre-renal causes of AKI?
Volume depletion (e.g., hemorrhage, diarrhea), reduced effective circulating volume (e.g., heart failure), and renal hypoperfusion (e.g., renal artery stenosis).
What is a renal cause of AKI?
Injury that lies within the kidney itself.
What are some examples of renal causes of AKI?
Ischemic injury, nephrotoxic injury (e.g., antibiotics), immune-mediated injury, and vascular disease.
What is a post-renal cause of AKI?
Obstruction to the lower urinary tract.
What are some examples of post-renal causes of AKI?
Renal stones, tumors compressing the urinary tract, blood clots, and enlarged prostate.
What are common clinical features of AKI?
Non-specific symptoms (nausea, lethargy), decreased urine output, fluid overload, electrolyte imbalances, and accumulation of toxic wastes.
What is the goal of pharmacological management in AKI?
To treat the underlying cause of AKI.
What fluid management strategies are used in AKI?
Fluid replacement for hypovolemia, IV fluids, blood transfusion for hemorrhage, and diuretics like furosemide for fluid overload.
What is renal replacement therapy?
A treatment for AKI when supportive therapies fail, involving methods like dialysis.
What are nephrotoxic drugs?
Drugs that can worsen AKI and should be stopped, such as NSAIDs and certain antibiotics.
What is Chronic Kidney Disease (CKD)?
A progressive and irreversible loss of renal function as a result of sustained renal injury.
What is the glomerular filtration rate (GFR) threshold for CKD?
A GFR of less than 60 ml/min.
What are the stages of CKD?
Stage I: Normal function; Stage II: Mild damage; Stage III: Moderate damage; Stage IV: Severe damage; Stage V: End-stage disease.
What is the most significant risk factor for CKD?
Diabetes mellitus.
What are common clinical features of CKD?
Impairments in creatinine and urea clearance, fluid and electrolyte balance, and effects on multiple body systems.
What are the signs of Stage I CKD?
Normal GFR (>90 ml/min) with no symptoms, but hypertension may be common.
What are the signs of Stage V CKD?
Severe symptoms with GFR < 15 ml/min, including complications from previous stages.
What systemic diseases are associated with CKD?
Hypertension, acute kidney injury, chronic glomerulonephritis, chronic pyelonephritis, and obstructive uropathies.
What are common electrolyte imbalances in AKI?
Hyperkalemia, which can lead to arrhythmias and cardiac arrest.
What is metabolic acidosis in the context of AKI?
An acid-base disturbance resulting from the accumulation of toxic wastes.
What happens to plasma creatinine levels when GFR decreases?
Plasma creatinine levels increase as there is no regulatory adjustment.
How does decreased GFR affect urea levels?
Urea levels increase because urea is both filtered and reabsorbed.
What compensatory mechanism maintains sodium levels until late-stage disease?
Compensatory mechanisms increase sodium excretion.
What are the consequences of sodium and water retention due to decreased GFR?
Oedema, hypertension (HPT), and heart failure can occur.
What condition may develop when GFR decreases to 20%?
Metabolic acidosis develops due to decreased H+ removal and HCO3- reabsorption.
What causes hypocalcemia in chronic kidney disease?
Impaired renal synthesis of vitamin D3 leads to decreased calcium absorption from the GIT.
What is the effect of hyperphosphatemia on calcium levels?
Hyperphosphatemia binds calcium, further contributing to hypocalcemia.
What are renal osteodystrophies?
They are conditions resulting from the combined effect of hyperparathyroidism and vitamin D deficiency.
What is a common metabolic consequence of chronic kidney disease?
Hyperlipidemia due to decreased removal of LDL.
What cardiovascular issues are associated with chronic kidney disease?
Hypertension, left ventricular hypertrophy (LVH), congestive heart failure (CCF), and ischemic heart disease (IHD).
What pulmonary complications may arise from chronic kidney disease?
Pulmonary edema, congestive heart failure (CHF), and metabolic acidosis leading to Kussmaul breathing.
What causes normochromic, normocytic anemia in chronic kidney disease?
Decreased erythropoietin (EPO) and reduced RBC lifespan due to uremia.
What are the effects of uremia on the immune system?
Suppression of the immune system, increased risk of infections, and virus-associated cancers.
What neurologic symptoms are common in chronic kidney disease?
Headaches, sleep disorders, impaired concentration, memory loss, and peripheral neuropathies.
What gastrointestinal symptoms are associated with chronic kidney disease?
Nausea, anorexia, vomiting, diarrhea, and bleeding ulcers due to uremic gastritis.
What is uremic fetor?
A type of bad breath caused by the breakdown of urea by salivary enzymes.
What hormonal changes occur in the endocrine and reproductive systems due to chronic kidney disease?
Decreased male and female sex hormones, reduced libido, and fertility.
What skin changes are observed in chronic kidney disease?
Sallow skin color, pallor, haematomas, ecchymosis, pruritus, and nail changes.
What is the role of erythropoiesis-stimulating agents in treating chronic kidney disease?
They are used to manage anemia.
What are phosphate binders used for in chronic kidney disease?
To treat hyperphosphatemia.
What is the purpose of sodium polystyrene sulfonate (Kayexalate)?
To treat hyperkalemia.
What is the standard dosing regimen for the combined oral contraceptive pill?
One tablet daily for 21 days followed by a 7-day pill-free break.
What are the contraindications for combined hormonal contraception?
Age ≥ 35 years and smoking ≥ 15 cigarettes per day, history of thromboembolic disease, and hypertension ≥ 160/100 mmHg.
What are the potential adverse effects of hormonal contraception?
Venous thromboembolic disease, arterial disease, hypertension, and irregular bleeding.
What is the mechanism of action of combined hormonal contraception?
Inhibits ovulation by suppressing LH and FSH.
What are the administration routes for hormonal contraception?
Oral, transdermal patch, vaginal ring, intramuscular, subdermal implant, and intrauterine system.
What is menopause?
A natural process of aging characterized by 12 consecutive months of amenorrhoea and permanent cessation of menses.
What is the average age of menopause?
51 years, with a range of 40 to 60 years.
What hormone is primarily produced by the ovaries before menopause?
Oestradiol, which is the most potent form of estrogen.
What happens to estrogen levels during menopause?
There is a loss of ovarian function leading to decreased secretion of estrogen and progesterone, resulting in increased gonadotrophins.
What are some symptoms of early menopause?
Mood disturbances, insomnia, hot flashes, irregular menstrual cycles, and headaches.
What are potential consequences of estrogen loss in postmenopause?
Increased risk of cardiovascular disease, osteoporosis, Alzheimer's-like dementia, and colon cancer.
What is the purpose of Hormone Replacement Therapy (HRT)?
To alleviate menopausal symptoms and reduce postmenopausal osteoporosis.
What are the two types of estrogen used in HRT?
Natural estrogens (like estradiol) and synthetic estrogens (like ethinylestradiol).
How is HRT administered?
Orally, transdermally, or topically, with options for cyclical or continuous preparations.
What are the recommendations for HRT usage?
Use the minimum effective dose for the shortest duration, and it does not provide contraceptive cover.
What are some risks associated with HRT?
Increased risk of endometrial, breast, and ovarian cancer, particularly with prolonged use.
What is osteoarthritis (OA)?
A degenerative joint disease characterized by progressive cartilage damage and loss.
What are common risk factors for developing osteoarthritis?
Older age (40+), history of joint injuries, strenuous jobs, being overweight, and genetics.
What are the clinical features of osteoarthritis?
Joint pain, stiffness, bony swelling, and asymmetry in joint involvement.
What mnemonic can help remember the signs and symptoms of OA?
OSTEO: Outgrowths, Sunrise stiffness, Tenderness, Experience grating, Only joints affected.
What is the goal of pharmacotherapy for osteoarthritis?
To reduce pain and inflammation.
What types of medications are used for osteoarthritis pain relief?
Nonopioid analgesics, NSAIDs, topical treatments, corticosteroid injections, and sodium hyaluronate injections.
What are common adverse effects of HRT?
Postmenopausal bleeding, acne, thrombophlebitis, headaches, hypertension, and weight gain.
What are the characteristics of osteoarthritis?
Progressive cartilage damage, narrow joint space, bony hypertrophy, and subchondral bone sclerosis.
What imaging techniques are used to assess osteoarthritis?
Clinical assessment and radiologic studies; newer imaging like compositional MRI shows promise.
What is the significance of Heberden's and Bouchard's nodes?
They are bony swellings in the hands indicative of osteoarthritis.
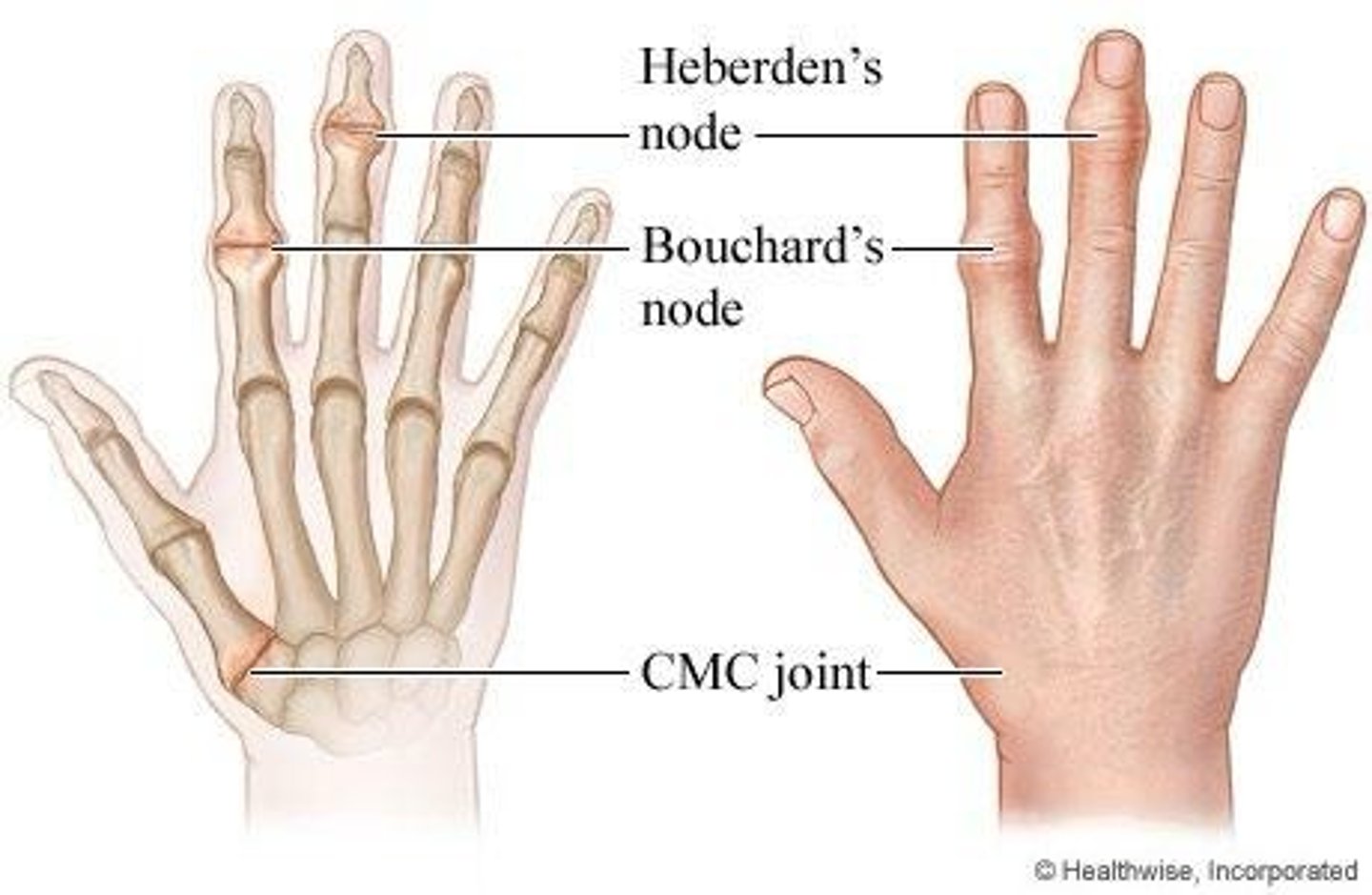
What are the symptoms of midmenopause?
Vaginal atrophy, increased infections, painful intercourse, and sexual disinterest.
What are the symptoms of postmenopause?
Increased risk of cardiovascular disease, osteoporosis, and Alzheimer's-like dementia.
What is the relationship between HRT and cardiovascular disease?
No increased risk for women taking estrogen alone, but a small increase for those on combined therapy.
What is the effect of oral HRT on stroke risk?
There is a small increased risk with oral preparations, but no risk with transdermal routes.
What is the impact of estrogen deficiency on the body?
Leads to vasomotor symptoms, genitourinary atrophy, and osteoporosis.
What is the typical age range for the onset of menopause?
40 to 60 years.
What is the significance of the first-pass metabolism in HRT?
Oral administration of HRT is subject to first-pass metabolism, which affects hormone levels.
What are NSAIDs?
Nonsteroidal anti-inflammatory drugs used to relieve pain and inflammation.
What properties do NSAIDs possess?
Analgesic, anti-inflammatory, antipyretic, and anti-platelet inhibition (e.g., Aspirin).
How do NSAIDs work?
They inhibit the synthesis of prostaglandins by blocking cyclooxygenase enzymes (COX-1 and COX-2).
What is the role of COX-1?
COX-1 synthesizes protective prostaglandins that regulate gastric acid secretion and renal blood flow.
What is the role of COX-2?
COX-2 is formed after tissue injury and promotes inflammation.
What are the effects of nonselective NSAIDs?
They block both COX-1 and COX-2, reducing inflammation but may cause GI irritation and bleeding.
What are selective COX-2 inhibitors?
Drugs that specifically inhibit COX-2, such as Celecoxib (Celebrex).
What are common adverse effects of NSAIDs?
Gastrointestinal issues, renal impairment, and cardiovascular problems.
What is osteoporosis?
A skeletal disease characterized by low bone density and increased fracture risk.
What causes osteoporosis?
Imbalance in bone resorption and formation, leading to loss of bone density.
What are the risk factors for osteoporosis?
Low calcium, age, lifestyle factors, gender (female), genetics, and certain medications.
What mnemonic can help remember the signs and symptoms of osteoporosis?
FRAIL: Fractures, Rounding of the upper back, Asymptomatic until fracture, Inches of height lost, Low back pain.
What is DXA?
Dual X-ray absorptiometry, a method to measure bone density.
What are common treatments for osteoporosis?
Calcium and vitamin D therapy, bisphosphonates, SERMs, and calcitonin.
What is the mechanism of action of bisphosphonates?
They inhibit osteoclast activity, reducing bone resorption and increasing bone density.
What is the prototype drug for bisphosphonates?
Alendronate (Fosamax).
What are the adverse effects of alendronate?
GI irritation, nausea, vomiting, and potential pathologic fractures with long-term use.
What precautions should be taken with bisphosphonates?
Patients with esophageal abnormalities, renal impairment, or hypersensitivity should use caution.
What interactions should be noted with alendronate?
Calcium, iron, and certain antacids can interfere with absorption.
How should alendronate be administered?
On an empty stomach with plain water, remaining upright for at least 30 minutes afterward.