CRNA interview Final
1/307
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
308 Terms
ARDS
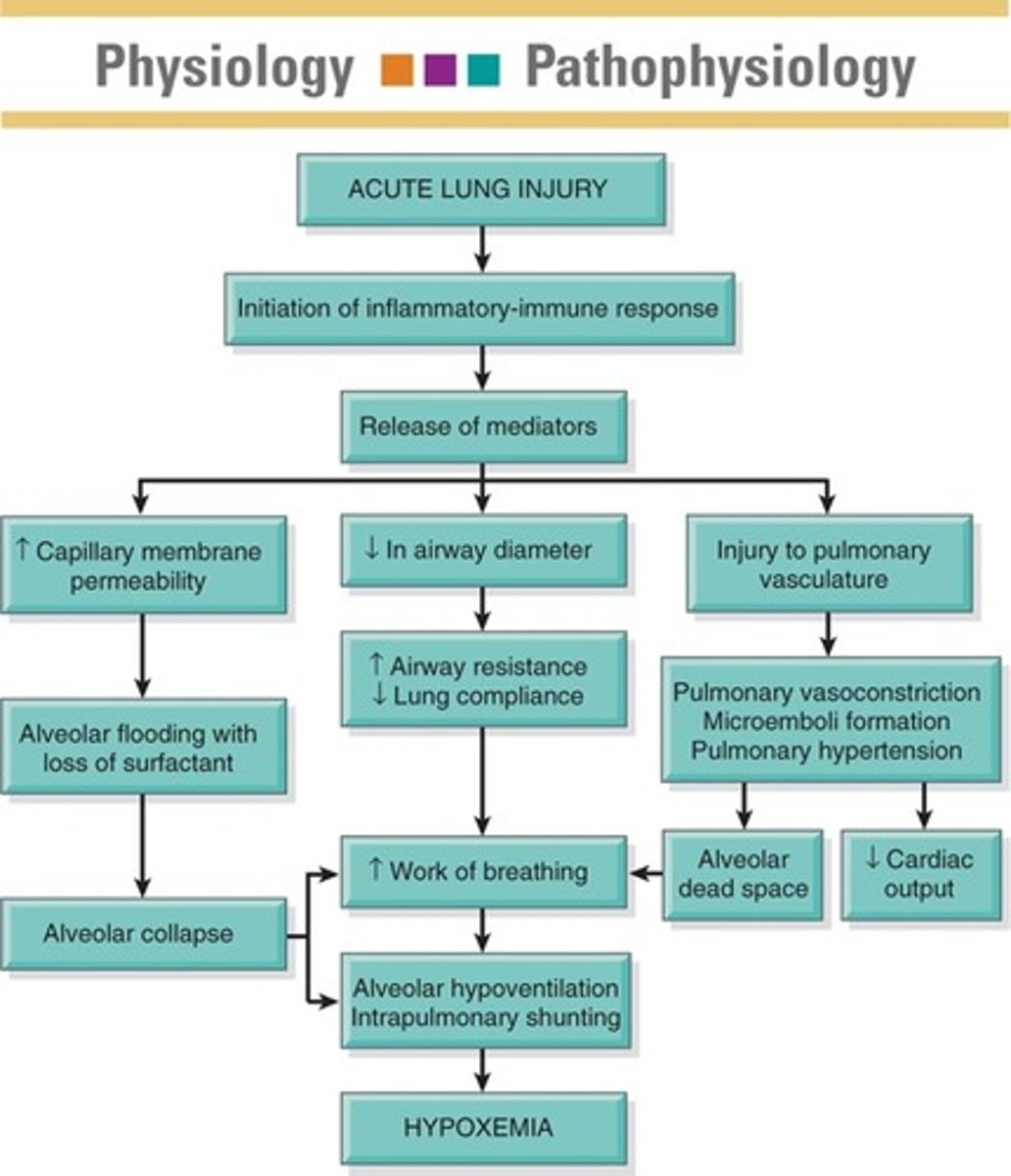
Pathophysiology-Preceded by acute lung injury causing inflammation, leading to fluid seepage in the lungs, decreased oxygen reaching the bloodstream, and shunting resulting in arterial hypoxemia despite high inspired oxygen levels. Acute respiratory distress syndrome in defined as acute diffuse lung injury caused by inflammation which refractory hypoxemia.
Many patient's I've cared form experienced ARDS due to Sepsis, COVID or Pneumonia.
Patho: Occurs in three phases:
1) exudative:
Damage to pulmonary capillary beds and alveoli leads to atelectasis and decreased lung compliance. (hypercarbia and hypoxia d/t increased pulmonary dead space)
2) Proliferative phase:
interstitial inflammation and damage persist. Lung is attempting to heal self
3. Fibrotic stage
Some patients progress to the fibrotic stage. Essentially scared lungs with decreased lung compliance for life.
Atelecstasis leads to severe hypoxia and poor lung compliance
Diganosis: Refractory hypoxemia, graded by using begin criteria and the P/F ratio
Treatment: treating underlying cause, mechanical ventilation with PEEP and Low Vt. Prone, Paralyze)
ARDS= ALI occurs leading to inflammatory immune response --> release of cytokines (TNF-a, IL-1, IL-6, IL-8)[SIRS] which attract neutrophils to the lungs. Neutrophils release reactive oxygen species and proteolytic enzymes--> causes DAMAGE to alveolar epithelium and endothelium (damage to endothelium triggers clotting cascade) --> pulmonary edema and hyaline membrane forms
1) Increased alveolar epithelial permeability and increased pulmonary endothelial permeability leading to alveolar flooding
2) Since alveolar epithelial cells injured, Surfactant decrease, atelectasis and shunting occurs (alveolar collapse). Fluid reabsorption inhibited
3) Impaired Gas Exchange & Hypoxia, increased dead space, pulmonary HTN and decreased compliance occurs
sepsis:
Sepsis can be defined as life-treating end organ dysfunction caused by dyssregulated immune response
Septic Shock: occurs when sepsis results in severe circulatory, metabolic and cellular dysfunction.
Patho:
Overcompensated immune systems reaction to infection which results in systemic cytokine release.
a. widespread vasodilation and fluid leakage fro capillaries (fluid leakage into interstitial space): impairs oxygen perfusion
b. Activated coagulation and formation of microemboli the reduce blood Flow and further impairs oxygen supply. CO2 build up and waste build up.
Body begins to leverage anerobic metabolism (metabolized glucose for energy and lactic acid is biproduct)
Treat:
1. Treat infection
2. Ensure end organ perfusion (
MAP > 65) 30cc/kg fluid bolus
3. Use of vasopressor support (levo, then vaso, then epi)
4. Monitor for organ dysfunction (AKI, ARDS)
Life-threatening condition caused by dysregulated immune response to infection, leading to excessive release of pro-inflammatory cytokines, widespread inflammation, and organ dysfunction.
Inflammatory Mediators Contributing to Septic Shock:
Interleukin-1: Released by macrophages and lymphocytes. produces fever, vasodilation and hypotension, edema, elevates WBC
Tumor Necrosis Factor-alpha: released by macrophages, natural killer cells, and mast cells. same effect as IL-1
Platelet-Activating Factor: released from phagocytes, platelets. Contributes to widespread clotting, generates same sx as IL-1 and TNF-aplha
OR
SIRS= a systemic inflammatory response from an insult to the body; causes a cellular reaction that begins a number of mediator-induced inflammatory and immune responses. 2 or more of the following:
Temp >38 or <36
HR > 90 beats/min
RR >20/min or PaCo2 <32
WBC >12,000, <4000, >10% immature bands
Sepsis= A manifestation of SIRS in response to an infection
Can be bacterial, viral, or fungal
Severe Sepsis= when sepsis causes altered perfusion to vital organs "end-organ dysfunction
Organ dysfunction or failure occurs from hypoperfusion and microvascular thrombosis
-Hypotension
-Lactic acidosis >2mmol/L
-Oliguria and increased creatinine
-Acute change in mental status
-Tachypnea and hypoxia
-Thrombocytopenia
-Elevated liver enzymes and coagulopathies
Septic Shock
Sepsis with hypotension despite fluid resuscitation
A serum lactate of >4 is considered as high risk as continued hypotension so it is in the same septic shock category
-Measure lactate within 3hrs, obtain blood cultures prior to starting antibiotics, 30ml/kg crystalloid
-Within 6 hours vasopressors for hypotension not responsive to fluids, CVP and ScvO2 (CVP should be 12-15)
Multiple organ dysfunction
The worsening progression of the body's inflammatory response, cascade of inflammatory mediators, endothelial injury, altered perfusion, microcirculatory failure
Treat cause of MODS
IABP
Indications: 1) LV Dysfunction, Unloading for mechanical complications after MI, Mitral regurgitation, ventricular septal rupture, MI with Shock, HF to bridge to transplant
A catheter that is placed in the femoral artery during the CVOR, cardiac Cath, or bedside (emergencies). IAB cath is placed in descending thoracic aorta , 1-2 cm below the L subclavian artery & above the renal artery branches.
Visibily on xray at 2 and 3rd ICS
Provides physiologic assistance to heart by decreasing oxygen demand and increasing coronary perfusion by allowing blood flow to the coronary arteries
-used short term in shock or heart failure
-dilates when heart is in diastole (relaxing) to pump blood to the coronary arteries, deflates when heart in systole (contracting)
It is counter-pulsation therapy. it works by inflating a helium filled balloon that rests in the thoracic arota and terminates before the L subclavian artery . The balloon inflates during diastole to provide increased blood flow to the coronary arteries (augmented diastole) as well as deflating prior to systole to lower after load. It lowers after load by displacing blood when it inflates and essentially creating a vacuum like effect in the space the blood previously was in and thereby reducing after load on the LV and allowing the LV to eject blood with less exertion.
if iabp will not inflate via machine attach 40ml syringe and stopcock and manually inflate every 5 mins
minimal CO assistance- provides only increase .5-1L
1) Reduces impedance of LV ejection (unloads heart) by rapid deflation just before systole
2) Increases diastolic coronary perfusion pressure by rapid inflation just after aortic valve closure with improvement in coronary diastolic flow
3) In RV failure, improvement is accomplished by improving right coronary perfusion pressure and reducing RV afterload by decreasing LV preload
diastole inflated --> coronary perfusion
improve myocardial oxygenation
increase cardiac output and organ perfusion reduction in left ventricular workload
CI:Aortic Regurgitation,Aortic Dissection, vascular Atherosclerosis, Sepsis
Complications: 1) Unipolar pacing - atrial spike interpreted as QRS leading to inappropriate inflation, Thrombocytopenia, Distal ischemia. If migrates: towards the heart can obstruct the left subclavian or left common carotid. if it migrates distally it will occlude renal artery. You have a pulse ox on left hand to monitor for subclavian obstruction, monitor loc for carotid obstruction and monitor urine output for renal artery obstruction. infection, emboli
verify placement with external marker, chest xray, or fluoroscopy. Tip of IABP should be 1-2 cm distal of left subclavian. on chest xray it is about level of carina. there is no radiopaque marker for distal end of balloon.INFLATE:When IABP console recognizes dicrotic notch (aortic value closure), at the peak T wave the balloon will inflate during diastole
DEFLATES: When IABP identified R wave, balloon with deflate. Deflates at systole which decreases after load , decreases LV workload, and decrease myocardial o2 demand
IV heparin therapy should be d/c’d 4 hours before removal
If the pt codes Place trigger mode on arterial pressure or internal trigger mode . Leave the pump running
S/S balloon rupture: Rust colored flakes of blood in the IABP line. stop the pump and place the pt in the trendelenberg position
Beta 1
Locations: postsynaptic cells in Cardiac muscle
MOA:-increase cyclic aMP which increases HR, contractility and AV nodal conduction. coupled to g's proteins which activate adenylyl cyclase to form cAMP, increases Ca in cells - > more Ca to bind to troponin and increase contractility / strength
B1 selective: Esmolol and lopressor
Atenelol, Bioprolol, Acebutolol
- increase in contractility (increase force of myocardial contraction)
- increase HR
- with the above, stroke volume and CO should increase
- excess=arrhythmia
Beta 2 effects
Location: Lungs and peripheral arterioles
vasodilation of the blood vessels, glycogenolysis (insulin release) of the liver, decreased motility of the GI muscles, relaxation of the uterus, dilation (relaxation) of the bronchial muscles
Dilates and relaxes smooth muscle in bronchials and dilates airways
Alpha 1 receptors
Location: Located in Lungs & peripheral arterioles
-increase calcium in muscle cells and leads to smooth muscle contraction (peripheral vasoconstriction)
vasoconstriction of vascular smooth muscle and artieal resistance
mydriasis
- More responsive to Norepi than Epi
- Activation causes vasoconstriction
- Abundant in vascular smooth muscle
- GI & urinary sphincters
- dilator muscle of the iris (reducing tone enlarges the pupils)
- arrector pili muscle of the hair follicles (reducing tone causes hair to stand on end)
Lungs and Smooth Muscle
- Vascular and nonvascular smooth muscle relaxation
Tx of asthma and premature labor (selective beta 2 agonists)
Postsynaptic receptors- on VASCULAR smooth muscle
activation of alpha 1 receptor causes vasoconstriction and increase in PVR and systemic arterial blood pressure
Alpha 2 effects
Locations: Brain
Arterial vasodilation
decrease insulin release
sedation
analgesia
inhibition of NE and Epi in brainstem
decreased SNS
Ex: Clonidine and Precedex
Presynaptic - activation of these receptors inhibits the release of NE (decrease sympathetic nervous system activity)
NE acts at presynaptic alpha 2 receptors to inhibit its own release
Example Alpha 2 agonist: Precedex
Hypovolemic Shock
Shock caused by fluid loss (hemorrhage, plasma via burns, or interstitial fluid via DI, emesis, diarrhea, diuresis); begins when intravascular volume has decreased about 15%
Initially compensated via increased HR and increased SVR boosting both CO and tissue perfusion pressures (MAP). RAAS is also activated to ultimately release ADH to retain H2O and aldosterone to retain sodium.
If hypovolemia persists, compensation will fail to result in decreased tissue perfusion (cardiogenic shock) resulting in increased lactate production from anaerobic metabolism etc.
SSX: poor skin turgor, thirst, oliguria, increased HR, thready pulse; and AMS
Signs from the Swan Gods:
High: SVR, HR
Low: CO/CI, CVP, PAWP
PAP may be normal
Treatment: Fluids (crystalloids), pRBC
Obstructive Shock
Shock that occurs when there is a physical obstructing blocking blood flow in the heart or great vessels, causing an insufficient blood supply to the body's tissues. leads to decreased venous return and/or excessive afterload (i.e., the force that the left ventricle has to overcome to eject blood through the aortic valve), resulting in decreased cardiac output.
There are two major causes of obstructive shock: a blockage of the pulmonary vascular system, thereby affecting the blood flow from the right-sided heart chambers to the left-sided heart chambers, as seen in significant pulmonary embolisms and severe pulmonary hypertension; or an extrinsic mechanical compression of the great vessels of the heart that alters the heart's cardiac output. Examples of extrinsic mechanical compression include a tension pneumothorax, pericardial tamponade, restrictive cardiomyopathy, and constrictive pericarditis.
Individuals with obstructive shock typically experience respiratory distress and may present with tachycardia, hypotension, tachypnea, air hunger (i.e., an extreme need to breathe), and chest pain.
A characteristic sign of causative conditions like pneumothorax and tamponade may include dilated and engorged neck veins, resulting from the inability of blood to return to the heart. Various constellations of signs may indicate a probable underlying cause. For example, hyperresonant sounds on percussion, distended neck veins, and absent breath sounds are typically indicative of tension pneumothorax. Distant heart sounds and decreased pulse pressure can usually indicate cardiac tamponade. Tension pneumothorax can mimic the signs of cardiac tamponade, with findings of distended neck veins and hypotension.
The initial treatment for obstructive shock requires resuscitative measures and ABC (airway, breathing, circulation) assessment, which includes su
From PE, tension pneumo, cariac tamponae, pericardidits
increase HR, SVR
decreased: CO, SvO2
Distributive Shock (Neurogenic, Anaphylactic, Septic)
- vasoDilatory shock, refers to systemic vasodilation and decreased blood flow to vital organs such as the brain, heart, and kidneys (inadequate tissue perfusion). It can also cause fluid to leak from the capillaries into the surrounding tissues as a result.
-Can be septic, anaphylactic and neurogenic
Anaphykatuc: caused by an increased level of immunoglobulin E (IgE) antibodies forming due to exposure to an allergen. IgE attaches to the surface of mast cells and basophils. Once the individual is exposed to the same allergen a second time, histamine is released from the mast cells and basophils, leading to systemic vasodilation and capillary fluid leak, thereby resulting in distributive shock.
-HR increased, CO variable (will be increased early on), ventricular filling pressures decreased, SVR decreased,
Widespread vasodilation resulting in decreased tissue perfusion.
Neurogenic - results from parasympathetic overstimulation and sympathetic under stimulation.
Anaphylactic - Widespread hypersensitivity reaction causing massive vasodilation.
Meds:epinephrine IM 0.01 mg/kg (max of .5 mg per dose), 1-2 L of NS, adjunctive: Benadryl, steroids, bronchodilators (albuterol)
Septic Shock:
Begins w an infection that progresses to bacteremia then SIRS w sepsis, then. severe sepsis, and finally MODS.
Very high mortality rate and can be caused by any class of microorganism. Common sources of infection are the lungs, urinary tract, GI, wounds, and vascular catheters.
Bacteria enter the bloodstream to produce bacteremia. Bacteria may directly stimulate an inflammation response or release endo/exotoxins into the bloodstream. This activates toll-like receptors on macrophages which trigger complement, coagulation, kinins, and inflammation cells.
The release of inflammatory cells triggers the release of secondary mediators such as cytokines, prostaglandins, platelet activation, oxygen free radicals, and NO. The inflammation process causes widespread vasodilation especially due to NO release. Early on we can see increased CO and HR, but later on IL, and other mediators start to depress myocardial function. Further damage to perfusion can be caused by the clotting cascade.
Treatment; vasopressors, EARLY antibiotic use, CRRT
Numbers from the Swan Gods:
High: CO/CI (early), HR, (early)
Low: CO/CI (late), SVR, PAWP, CVP
Cardiogenic shock
Pump failure
Decreased CO w. evidence of tissue hypoxia in the presence of adequate blood volume
most cases follow an MI, but can also be caused by L HF, dysrhythmias, valve dysfunction, PE, septal rupture, myocardial or pericardial infections, tamponade (obstructive type shock), drug toxicity (Beta Blocker)
SSX: AMS, SOB, increased RR, increased edema, dusky skin, marked hypotension, oliguria, ileus, tachycardia
How the body hates itself: for compensation RAAS is activated as well as release of catecholamines (epi, norepi) causing increased contraction and increased SVR and HR. These mechanisms increased myocardial oxygen demand and need for nutrients. This will further strain the heart resulting in further damage. Sometimes this is what even leads to shock.
Numbers from the Swan Gods:
Increased SVR, PAWP, PAP, CVP
Decreased CO, CI, BP
Treatment: Inotropes, vasopressors (ABP, impella, early PCI or bypass to minimize damage.
normal tidal volume,
FRC,
fucntional residual volume,
Vital capacity,
tidal capacity;
minute ventilation
TV- ; volume of air inhaled or exhaled in a normal breath
IDW 6-8 mL/kg normal. 4-6 ml?kg in ARDS
Min ventilation: total volume of air inhaled and exhaled each minute
MV = RR x Vt. Normal 5-8L/min
inspiratory capacity- 3.8L (IRV+ TV)
Inspiratory reserve volume- 3.3L; maximal inspiration above the tidal volume
Expiratory reserve volume- 1.1L; additional volume expired below the tidal volume.
residual volume- 1.2 L; volume of gas remaining after a maximal forced expiration
Functional Residual Capacity (FRC) - 2.4L( ERV+TV); volume remaining in the lungs after a normal tidal volume is expired.
Total lung capacity -6.0L
VItal Capacity (VC): Amount of air exhaled after a maximal inspiration. Normal ~4800mL
Spo2 mechanism and beer lambert law
The Beer-Lambert law describes the attenuation of light with the properties of the material through which the light is traveling. Beer's Law states that the concentration of a chemical solution is directly proportional to its absorption of light ie the concentration of oxy pr doxyhemaglobin. The Beer-Lambert law enables us to measure SpO2 by using the molar extinction coefficients of HbO2( oxygenated) and RHb ( deoxygenated).
reflective( forehead) and transmissive (everything else pulse ox.
-Oxygenated blood absorbs more infrared light while deoxygenated blood absorbs more red light. Two waveforms of light are emitted from one diode through tissue to another diode. A ratio of the change in signal is compared to a chart and produces a reading.
Beta Blockers
Located:SA node, atria, AV node, ventricles, skeletal muscle, bronchial smooth muscle
noncardioselective: Works on B1 and Beta 2 by dilating blood vessels, decreasing tremors, and relax smooth muscle in airway
Propranolol
Nadolol
Labetalol
Carvedilol
Sotalol
cardioselective beta blockers
metoprolol, atenolol, esmolol
CI: Asthma, cocaine use (know
why this is-unopposed alpha)-can exasperate coronary vasospasm due to alpha adrenergic receptors, hr less than 60, decreased BP..
asthma(causes bronchospasm)
COPD(causes bronchospasm)
Chronic bronchitis (causes bronchoconstriction)
Emphysema(casues bronchoconstriction)
Bradycardia and AV block(second to third degree blockEffects: negative inotropy (decreased contractility)
negative chronotropy (decreased HR)
negative dromotropy (decreased conduction velocity)
decreased relaxation rate
mild vasoconstriction
BB Toxicity: bradycardia, ventricular dysrhythmia, torsades, HPN, CNS depression
Action Potential; What is an action potential?
Membrane potential:
When membrane potential depolarizes suddenly and repolarizes back to its resting state.
-If the membrane potential moves toward zero, that is a depolarization because the membrane is becoming less polarized, meaning there is a smaller difference between the charge on the inside of the cell compared to the outside. This is also referred to as a decrease in membrane potential. This means that when a neuron’s membrane potential moves from rest, which is typically around -65 mV, toward 0 mV and becomes more positive, this is a decrease in membrane potential. Since the membrane potential is the difference in electrical charge between the inside and outside of the cell, that difference decreases as the cell’s membrane potential moves toward 0 mV.
If the membrane potential moves away from zero, that is a hyperpolarization because the membrane is becoming more polarized. This is also referred to as an increase in membrane potential.
Na, K, and Ca;
Potassium has the largest influence on ionic membrane concentration
sympathetic nervous system
the division of the autonomic nervous system that arouses the body, mobilizing its energy in stressful situations
Agonists: EPI, NE, dopamine- increase HR, dilated pupils, DRYSLUDS
Antagonists: BB, alpha blockers (phentolamine)
Autonomic Nervous System and Somatic; What are they? Where is the SNS and PNS located?
SNS revs up the body for action, while PNS calms the body down after action.
Somatic nervous system is voluntary motor system under conscious control and relates to skeletal muscle. The neuro transmitter acetylcholine is released from presynaptic terminals of the motoneurons and activates nicotinic receptors on the motor end plates of skeletal muscles (this is where DNMB work). An action potential causes the muscle to contract prompted by acetylcholine.
Autonomic is involuntary.The part of the PNS that controls the glands and the muscles of the internal organs. Comprised of sympathetic and parasympathetic nervous system
SNS is found in the thoracolumbar outflow. PSN is located within the brain stem and sacral region/craniosacral outflow (CN 3, 7, 9, 10)
In the SNS, the pre ganglion releases acetylcholine > Ach stimulates the post ganglionic motor neuron > post ganglionic motor neuron releases NE or other catecholamines to stimulate the target tissue; Remember: pre ganglion is cholinergic (releases Ach); post is adrenergic in SNS
In the PNS, the pre ganglionic motor neuron releases Ach > Ach stimulates the post ganglionic motor motor neuron > post ganglionic MN releases Ach; Remember: pre ganglion is cholinergic and post ganglion MN is cholinergic
Extubation Criteria
indication for intubation and ventilation resolved
hemodynamiclly stable- 1 or no low dose pressor
fio2 > 40%
peep 5
free of agitation and confusion and can follow commands
iniate spontaneous breaths
using rapid shallow breathing index for eval to extubate which is respiratroy rate ratio to tidal volume. want it less than 105
Readiness testing for extubation includes the following
- Adequate Oxygenation (with FiO2 <40% and Spo2>90% and Peep <5)
- pH > 7.25
- MV less than 10L/min
- Spontaneous TV greater than 5-8 mL/kg
- Vital Capacity greater than 10ml/kg
- RSBI (TV/RR) less than 105 breaths/min/L
- Max negative Inspiratory pressure greater than -30cm water
-Fi02<40%
-Cause of RF has improved/resolved
-PEEP <5
-patient is hemodynamically stable
-patient has a cuff leak
-patient has a mental status that allows for handling of secretions and protection of one's own airway. Patient can lift head off of pillow
-patient is calm/comfortably breathing. RSBi<105 where RSBi = RR/Fi02 .. Ie 20/.4
-MIP more negative than -30
-Acid Base is neutral
-Can the patient cough on command?
GABA; What is GABA?
inhibitory neurotransmitter within the CNS
Can be either GABA A or B subunit. GABA function is to reduce the activity of the neurons to which it binds. It is estimated that 40% of the synapses in the human brain work with GABA and therefor have GABA receptors. They are channel receptors, so when GABA binds to them, they change shape slightly to allow ions to pass through their central channel (mostly negative ions like Cl, which then reduces its excitability). Because of this, GABA is considered an inhibitory neurotransmitter (as opposed to excitatory neurotransmitter like glutamate- which augment nerve impulses in the neuron).
GABA A: fast synaptic inhibition. Once recpeotr binds to GABA. It allows Cl- to move across membrane and follow a positively charged ion into the intracellular space. The addition of negative charge will decrease the resting potential of the cell, thus causing an inhibitory effect. GABAa receptors are located throughout the central nervous system. However, they have high concentrations in the limbic system and the retina. and hyperpolarizes (inhibits) the postsynaptic cell; this is on the site of Benzos and barbiturates. Gaba A receptors is an Ionotropic receptor- Cl passes through and hyperpolarize neuron and make it less likely to potentiate an action potential
GABA B:slow inhibitor increases K conductance and hyperpolarizes the postsynaptic cell. After GABA has bound to the receptor, potassium conductance is increased. Adenylyl cyclase is activated, which prevents calcium entry thus inhibits presynaptic release of other neurotransmitters. GABAb locations include the thalamic pathways and cerebral cortex. Gaba B receptors are Metabotropic receptors causing opening of K channels - K flows OUT of neuron channel and hyperpolarizes the neuron making it less likely to potentiate an action potential
GABA receptors-respond when GABA is released into the post-synaptic nerve terminal. They are considered the chief inhibitory receptors for the central nervous system.
Muscarinic Receptors
Located in heart/ ganglia of peripheral parasympathetic NS. autonomic effector organs (heart, brain, exocrine glands, smooth muscle)
Muscarinic Receptors - M1-M5
M1 - CNS, parietal cells
M2 - Heart (SA, AV, Bundle of HIS), presynaptic nerve terminals
M3 - Exocrine glands, bronchioles, GIT, bladder (increased secretions)
M4 and M5 - CNS
located post-synaptically in the effector organs such as smooth muscle, cardiac muscle, and glands supplied by parasympathetic fibers. Activation causes parasympathetic response in respective organs.
-involved in peristalsis, micturition, bronchoconstriction, and several other parasympathetic reactions.
They are located in the CNS, especially the brain. Also found in the heart. Decreases HR.
Nicotinic Receptors:
Found in skeletal muscle. CNS, adrenal medulla, autonomic ganglia and neuromuscular fx of somatic muscles
Ach binds to nicotinic receptors on the neuron > Na channels open causing a more positive membrane within the neuron > the influx of Na (making neuron more positive) causes the neuron to reach threshold potential > voltage gated Na channels open causing a rapid increases of positive ions and prompts an action potential to take place within the neuron
Located: on all autonomic nervous system ganglia and the adrenal medullla at the neuromuscular junction found in skeletal muscle'
Nicotinic is fast response, muscarinic is slow and prolonged. Nicotinic mediates excitation in target cells, while muscarinic mediates inhibition and excitation in target cells. Nicotinic is presynaptic while muscarinic is pre and post synaptics. Nicotinic is an ion channel. NMBAs are classified according to their interaction with nicotinic Ach receptors (nAChRs) at the neuromuscular junction.
Response: Stimulation of parasympathetic and sympathetic postganglionic nerves and release of epi from the adrenal medulla.
-triggers rapid neural and neuromuscular transmission. and contracts skeletal muscle
Adrenergic
Adrenergic and cholinergic are two receptors in the autonomic nervous system. Adrenergic receptors work for the sympathetic nervous system
Dopamine Receptors
Adrenergic receptors found in renal tissue that when stimulated, relax the renal arteries and therefore increase renal perfusion.
d1- located in renal vasculature, mesenteric vasculature, cerebral and coronary beds
Cholingeric Recepotrs
Cholinergic receptors -One category of receptors in PNS (other is adrenergic). The receptors mediate responses to acetylcholine. They mediate responses at all junction where ach is the transmitter. Three main receptors: nicotinicN, nictonicM, and muscarinic
RAAS System: Renin-Angiotensin-Aldosterone System
Renin is a proteolytic enzyme that is released into the circulation by the kidneys.
Its release is stimulated by:
-sympathetic nerve activation (acting through β1-adrenoceptors)
-renal artery hypotension (caused by systemic hypotension or renal artery stenosis)
-decreased sodium delivery to the distal tubules of the kidney.
The release of renin is inhibited by atrial natriuretic peptide (ANP), which is released by stretched atria in response to increases in blood pressure.
juxtaglomerular (JG) cells associated with the afferent arteriole entering the renal glomerulus are the primary site of renin storage and release. A reduction in afferent arteriole pressure causes the release of renin from the JG cells, whereas increased pressure inhibits renin release. Beta1-adrenoceptors located on the JG cells respond to sympathetic nerve stimulation by releasing renin. Specialized cells (macula densa) of distal tubules lie adjacent to the JG cells of the afferent arteriole. The macula densa senses the concentration of sodium and chloride ions in the tubular fluid. When NaCl is elevated in the tubular fluid, renin release is inhibited. In contrast, a reduction in tubular NaCl stimulates renin release by the JG cells.
When renin is released into the blood, it acts upon a circulating substrate, angiotensinogen, that undergoes proteolytic cleavage to form the decapeptide angiotensin I. Vascular endothelium, particularly in the lungs, has an enzyme, angiotensin converting enzyme (ACE), that cleaves off two amino acids to form the octapeptide, angiotensin II
Ang 2 effect:
-Constricts resistance vessels (via AII [AT1] receptors) thereby increasing systemic vascular resistance and arterial pressure.this signalling occurs via a Gq protein, to activate phospholipase C and subsequently increase intracellular calcium.
-Stimulates sodium
1) Juxtaglomerular apparatus in the kidney senses decreased renal perfusion
2) Kidney releases renin from juxtaglomerular cells in response to decreased perfusion.
3) Angiotensinogen (released from liver) is converted to ANG I with the renin enzyme
4) ACE (released from surface of pulmonary and renal endothelium) is released and converts ANGI to ANGII
5) ANG II Effects: 1- increase sympathetic activity 2- tubular Na and Cl reabsorption and K excretion. H20 Retention. 3- Adrenal cortex releases Aldosterone (which increases H2O retention, tubular Na and Cl reabsorption and K excretion) 4- Arteriole vasoconstriction IOT increase BP. 5- Posterior Pituitary releases ADH (H2O reabsorption occurs in collection ducts)
6) Overall Net RAAS Effect: Water and salt retention. Effective circulating volume increases. Perfusion of juxtaglomerular apparatus increases.
OR
The renin-angiotensin-aldosterone system plays an important role in regulating extracellular fluid volume and sodium content. This is very important in the
control of blood pressure. Below is a summary of how each part of the system is
activated.
First:
• Renin is produced in the kidneys and converts angiotensinogen into angiotensin 1.
Second:
• Angiotensin converting enzyme (ACE) is produced in the lungs and then converts angiotensin 1 to angiotensin 2.
Third:
• Angiotensin 2 increases the release of aldosterone from the zona glomerulosa of the
adrenal cortex.
Fourth:
• Aldosterone then acts on the collecting duct of the kidney to increases the rate of
sodium reabsorption and potassium secretion.
ACE inhibitors
"PRIL" Captopril, Enalapril, Afosiopril, LisinoprilB
Antihypertensive. Blocks ACE in lungs from converting angiotensin I to angiotensin II (powerful vasoconstrictor). Decreases BP, Decreased Aldosterone secretions, Sodium and fluid loss. Some Alo used in CHF.
prevent the conversion of angiotensin I to angiotensin II which prevents vasoconstriction and retention of sodium and water
Check BP before giving (hypotension)
*Orthostatic Hypotension
inhibiting the formation of angiotensin 2
block the breakdown of bradykinin
produce vasodilation
SE: Cough, angioedma, reanal Insufficient
and cause vasodilation
decreased blood volume
decreased sympathetic activity
inhibit cardiac and vascular hypertrphy
Vent Settings
AC: Set tidal volume and RR. the pt also receives the set tidal volume for each breath triggered by the pt's spontaneous effort, all breaths are machine breaths, full vent support.- delivers preset TV, RR and flow rate
- patient cannot generate spontaneous volumes
- each patient generated respiratory effort over the above set rate is delivered the set volume and flow rate.
SIMV: Set tidal volume at the set breath rate and all breaths above the set rate are spontaneous breaths at the patient's own tidal volume. All machine breaths are synchronized with the patient's breathing effort, provides full or partial ventilatory support.,
-Machine breaths are synchronized w/ pt breathing effort
-Can decrease RR to allow for the patient to do more work
-Can be pressure supported
PS: Receives an increase in the airway pressure during inspiration to boost the spontaneous tidal volume, patient triggered mode, used frequently during weaning to reduce the work of breathing and to overcome imposed work of ett and ventilator circuit.,Mode of mechanical ventilation in which preset positive pressure is delivered with spontaneous breaths to decrease work of breathing
-Provides pressure during inspiration to increase TV
-Pt triggered
• Overcomes resistance of vent circuit and ETT during SPONTANEOUS breathing
CPAP: -PEEP in spontaneously breathing patient but continuous rather than at end expiration
-Pt does all of the WOB
-May offer pressure support
PC: -ventilator allows air flow into the lungs, until preset inspiratory pressure is reached
-Vt is variable
-PIP is better controlled
-pressure ventilation allows for delivering gas via pressure volume
-Useful for patients w/ poor lung compliance, ARDS, high PIPs
What is the difference between depolarizing and nondepolarizing
neuromuscular blocking agents (mechanism of action)- examples?
The key difference between depolarizing and nondepolarizing neuromuscular blockers is that depolarizing neuromuscular blockers act as acetylcholine receptor agonists while nondepolarizing neuromuscular blockers act as competitive antagonists.
a. Depolarizing: Succinylcholine
Depolarizing NMBA action produce skeletal muscle relaxation by binding directly to the nicotinic ach receptors to cause prolonged depolarization. (depolarize the receptor (phase 1)->no antidote for this phase --> then slowly decrease skeletal muscle activity (phase 2) by stimulating it for so long)
Ex. Succinylcholine
SE: Fasciculations (happens when a single peripheral nerve that controls a muscle is overactive, resulting in involuntary muscle movement), hyperK and Malignant Hyperthermia
Succinylcholine
More likely to cause malignant hyperthermia
Keep dantrolene at bedside
Contraindicated with burns, hyperkalemia, muscle traumaNondepolarizing: Rocuronium, Atracurium, Nimbex, Vec
Nondepolarizing act as COMPETITIVE ANTAGONISTS (compete with ACh for the binding site at the nicotinic Ach receptors), preventing the initiation of an action potential. (just sit in ach receptor and dont allow ach to bind to receptor). Steroidal and benzylisoquinolinium then they are classified by duration of actions (short, intermediate and long acting agents)
Calcium channel blockers
Calcium causes the heart and arteries to contract more strongly. By blocking calcium, calcium channel blockers allow blood vessels to relax and open thus causing vascular smooth muscle to relax to decrease muscle contractility
Block voltage-dependant L-type Ca2+ channels of cardiac and
they bind to L type calcium channels in smooth muscle, cardiac myocytes, and cardiac nodal tissue (SA/AV nodes).
You’ll see negative inotropy (decreased contractility)
negative chronotropy (decreased HR)
negative dromotropy (decreased conduction velocity)
vasodilation
Ex: nicardipine, verapamil, cardizem, nifedipine
Verapamil
Diltiazem
* These have effect on HR. Both cause some vasodilation but significantly decrease contractility, and also suppress AV node conduction slowing the heart rate
-* this is why cardene (nicardipine) is used for HTN emergency while cardizem (diltizem) is used for A-fib RVR
They block the influx of calcium into myocardium resulting in a decrease in contractility. It helps keep vasculature relaxed and decreases SA and AV node conduction. (decreases blood pressure, conduction, and contractility). calcium activates contractility in vascular smooth muscle
Bohr
The Bohr effect describes hemoglobin's lower affinity for oxygen secondary to increases in the partial pressure of carbon dioxide and/or decreased blood pH. This lower affinity, in turn, enhances the unloading of oxygen into tissues to meet the oxygen demand of the tissue.
a decrease in the amount of oxygen associated with hemoglobin and other respiratory compounds in response to a lowered blood pH resulting from an increased concentration of carbon dioxide in the blood.
Haldane
Ability of deoxygenated hemoglobin (a protein composed of an amino group) to carry more carbon dioxide (CO2) than in the oxygenated state
Oxygenation of blood in the lungs displaces carbon dioxide from hemoglobin which increases the removal of carbon dioxide.
what are acetylcholinesterase inhibitors and how do they work
Ex. neostigmine, pyridostigmine,
increase availability of Ach, Ach can compete with binding blocker and activate. They increase the availability of acetylcholine at the neuromuscular junction, to compete with nondepolarizaing NMBAs for nicotinic ach receptors and restore neuromuscular transmission
SE: can cause too much Ach -> too much activation of parasympathetic NS
can cause bradycardia, asystole, salivation, pupil constriction, increased UOP, peristalsis, bronchoconstriction
ostigmine preferred because higher affinity for acetylcholine receptors)
Ion channels that span the postsynaptic membrane, and they have extracellular, intramembranous and cytoplasmic portions. They mediate fast neurotransmission in the central and peripheral nervous system. It is the neurotransmitter at all autonomic ganglia (sympathetic and parasympathetic). There are two broad classes of cholinergic receptors, nicotinic and muscarinic (nicotine and muscarine mimic effects at of Ach at the respective receptor sites). Nicotinic receptors are what NMBAs interact with.
Preload
volume of blood in ventricles at end of diastole
To increase preload: IV fluids, blood, give pressors to vasoconstrict
To decrease: diuretics, nitrates, nitroprusside, morphine.
the volume/pressure in the ventricle at the end of diastole after the AV valve closes just prior to ejection
RV preload: CVP
LV preload: PAOP
Afterload
the pressure (resistance) against which the ventricle must pump to open the valve.
Pulmonic afterload: PVR- 100-250 dynes/sec/cm5
Aortic afterload: SVR- SVR) 800-1200 dynes/sec/cm5
measured by pulmonary (right ventricle) and systemic (left ventricle) vascular resistance
To decrease: nitroprusside, ACE inhibitors, hydralazine, calcium-channel blockers, IABP, Nitroglycerin (high doses)
Contractility:
Force of contraction (Inotropic)
To increase: dobutamine, dopamine 5-10mcg/kg/min
primacor, epinephrine drip
To decrease: negative ionotropes (beta blockers, calcium channel blockers)
Normal lab values
WBC
RBC
H&H
Platelets
Ca
Mg
WBC-4.5-11
RBC-male is 4.45-5.65 Female is 3.92-5.13,
H&H-male 13.2-16.6 female 11.6-15,
Platelet count-150,000-450,000,
Ca-8.5-10.2
Mg-1.7-2.2
DKA
potentially life-threatening complication in patients
with diabetes mellitus. DKA results when the body has a shortage of insulin, and responds by burning fats instead of sugars for energy. The byproducts of fat metabolism are ketones, which
are acidic in nature thus lowering pH, and are responsible for most of the
symptoms and complications. The serum blood sugar levels become very high which causes
glucose to spill over into the urine causing an osmotic diuresis. The person soon becomes
dehydrated and hypovolemic.
Causes with the diabetic patient:
• Pneumonia
• UTI
• Influenza
• Pregnancy
Signs/Symptoms:
• Metabolic acidosis
- pH < 7.35
- HCO3 < 22 mEq/L
- PaCO2 < 35 mmHg
• Blood glucose > 240 mg/dl
• Ketones in urine
• Acetone breath
• Extreme thirst
• Frequent urination
• Dry or flushed skin
• Kussmaul breathing (deep rapid breathing)
- Body's attempt to correct the metabolic acidosis by blowing off CO2
Treatment:
• Fluid replacement
• Insulin drip
• Potassium replacement
• Bicarbonate (fix metabolic acidosis)
what is hyperpolarization?
Hyperpolarization refers to a state where the potential across the membrane is more negative than the resting potential
-increases the stimulus required to move the membrane potential to the action potential threshold
what is depolarization?
Loss of a state of polarity; loss or reduction of negative membrane potential
-due to change in permeability and migration of Na ions inside the cell
CO
CI
SV
SVR (Systemic Vascular Resistance)
PAP
PAOP
PVR (pulmonary vascular resistance_
ICP
CPP
MAP
CVP
CO: 4-8L/min
HRx SV
CI: CO/BSA
Normal 2.5-4L/min/m2
SV: Stroke Volume: mL blood in 1 contraction. Determined by preload, afterload, and contractility
50-100mL
SVR: LV afterlaod. Normal 800-1200
(MAP- CVP)/CO x80
PAP: Systolic 15-30 mmHg. Diastolic 5-15 mmHg. Mean < 20
PAOP: 8-12 mmHg (varies depending on LV function)
PVR: RV afterload. 50 - 250
ICP: monitoring for symptoms like altered consciousness, headache, nausea, and changes in pupillary size. 5-15 mmHg
CPP: pressure of blood through the coronary circulation as a result of the pressure gradient between the aortic pressure and the right atrial pressure MAP- ICP. Normal 60-80
MAP: diastolic pressure + 1/3 pulse pressure
CVP: RA pressure or RV preload. Normal 2-6 mmHg
What is a inotrope
Force of contraction of heart
Postive ex: Dopa, epi, amio
NEgative: Labetolol, propanolol
what is a dromotrope
drugs that effects the conduction speed in the AV node
-example of negative dromotropes: verapamil
positive dromotropes: phenytoin
Chronotrope:
HR
negative chronotropes: digoxin, acetylcholine, metoprolol
Phosphodiasterase 3 receptor
Uses: Pulm HTN
breaks down cyclic aMP
-when inhibited you prevent the breakdown of cyclic aMP which then increase amount of cyclic aMP in cardiac cells and will increase HR, contractility and AV nodal conduction
-in peripherals it will lead to smooth muscle relaxation
Ex. Sildenafil, tadelafil
CRRT
Continuous Venous-Venous HemoDiaFiltration.
preferred sites for insertion- RIJ - Femoral - LIJ -SC
Effluent Dose (goal 20-25 ml/kg/hr delivered) =
Dialysis rate (ml/hr) + Predilution replacement rate (ml/hr) + Postdilution replacement rate (ml/hr) + Patient fluid removal (ml/hr)
Weight in Kg
purpose of countercurrent- it allows for the continuous maintenance of a diffusion gradient by continuously introducing fresh dialysate and blood to each other. eliminates the rate of diffusion decreasing at the end of the filter.
INDICATIONS: Usually used for patient who cannot tolerate iHD. For patients who are more hemodynamically unstable. The overall goal is to remove toxins, excess fluid, and achieve fluid balance. Used for patients who are:
unstable AKI,ARF, ESRD; elevated Cr and BUN; severe fluid and electrolyte imbalances; some acid/base imbalances; sepsis for cytokine clearance; rhabdomyolysis; CHF for fluid overload w/out diuretic response
Diffusion: Transport of a solute across a membrane, from a high to low concentration. effective for small molecules not large
Convection: Transport of a solute across a membrane along with a solvent via solvent drag. (kinda how water is dragged with Na). greater and faster flow increases clearance. Has to have replacement bc it requires large removal of fluid
predilution- decreases clotting but makes less efficient
post-dilution- increases clotting but makes clearance more effective
Adsorption-the process that molecules will start to cling to the filter membrane. Large molecules
Ultrafiltration: filtration through a semipermeable membrane, where small particles and macromolecules are separated from the body fluid water
Ultrafiltration is driven by a pressure gradient(osmotic, hydrostatic, oncotic) between the blood compartment and effluent compartment
can create positive pressure by blood pump(pushing) or the effluent pump ( negative )
PRESSURES:
Transmembrane Pressure (TMP): The hydrostatic pressure gradient across the membrane. The driving force that causes UF; high TMP w normal return suggest filter issue; High TMP w high return suggests either filter or return line issue.
Clogging of the membrane by proteins causes tmp to raise
Determinants- membrane pore permeability
-hydrostatic/oncotic pressure across membrane
-rate of replacement solution
-blood flow rates
Pressure drop (Δp):
reflects pressure conditions of hollow fiber