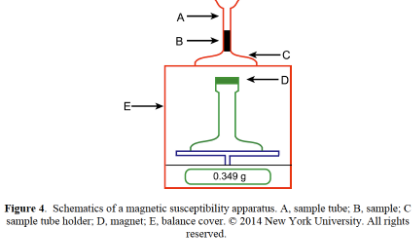
Magnetic Susceptibility
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
ferromagnetism
significant attraction to a magnetic field and capable of making magnetic field of its own
ex. iron, nickel, cobalt, -16°C gadolinium, other cold alloys
ordinary magnetism
all substances posses some ability to react to a magnetic field; 103 to 106 times weaker than ferromagnetism
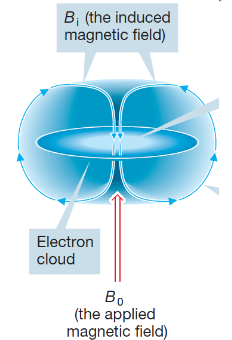
diamagnetic
magnetic dipole moment per unit volume is antiparallel to the field; paired electrons; present in all substances, no net mag. moment because all spins cancel out; temperature independent
in external field, induction causes internal current due to the change in the electron orbital angular momentum; this current opposes the ex. field
ex. bismuth
paramagnetic
magnetic dipole moment per unit volume is parallel to the field; unpaired electrons; permanent mag. moment; electron spins and orbital angular moments are not zero; temperature dependent
in external field, moments tend to line up with the mag. field; relatively small force
ex. odd # electrons, free atoms and ions with partially filled inner shell, molecular oxygen, organic biradicals, metals
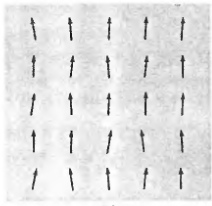
Ferromagnetic phenomena
exhibits a net magnetic moment (spontaneous magnetization) even in the absence of an external magnetic field; Electron spins (magnetic moments) are arranged in a regular (parallel) pattern due to magnetic interactions between them (nearest neighbor)
magnetic interaction
any dependence of the energy of 2 or more moments on their relative orientations; orginates from electrostatics not magnetism
no magnetic interactions in the absence of external field
Individual mag. moments point in random directions due to thermal motions, no net mag. moment
Antiferromagnetic phenomena
mag. moments (spins) are ordered in an antiparallel arrangement; no net total mag. moment (in the absence of an external field); magnetic interactions favors antiparallel orientation of neighboring moments
ex. MnO and Cr
Magnetism and temperature
spontaneous magnetization decreases with increasing temp. and vanishes above a certain critical temp.
So, ferro- and antiferromagnets display their properties below their critical temp.
Can magnetism be explained by classical/statistical mechanics?
NO! Classic system in thermal equilibrium cannot display a magnetic moment
partition function
describes the statistical properties of a particular system in thermodynamic equilibrium
Bulk Magnetism
induced magnetisation depends on strength of magnetic field
M=χB
M = magnetic moment per unit volume
B = external magnetic field
χ = magnetic susceptibility per unit volume/volume susceptibility/induced moment per unit volume per unit applied field
>0 for paramagnetic
<0 for diamagnetic
mass susceptibility
induced moment per unit mass per unit applied field (m3kg-1)
χw = χ/ρ
ρ = density
molar susceptibility
induced moment per mole per unit applied field (m3mol-1)
χw = ℳχw = Vχ
ℳ = molar mass
V = molar volume
Macro- and microscopic magnetic properties
magnetic moemt of atom results from
electron spin - paramagnetic
orbital angular momentum of electrons about the nucleus - paramagnetic
change in the orbital momentum induced by an external magnetic field - diamagnetic
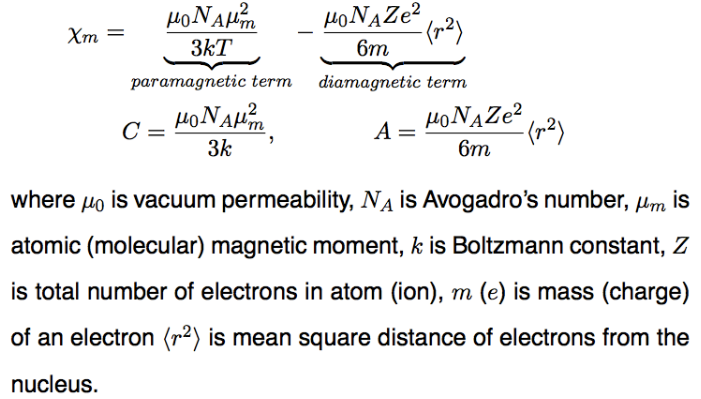
since diamagnetic term is so small, μm=797.1μBsqrt(Tχm)
assume μm=μS, μS=μBsqrt(n(n+2))
Hund’s rules
electronic orbitals are filled according to these; gorund electronic state characterised by
maximum value of the total spin S allowed by the exclusion principle
max. value of the total orbital angular momentum L consistent with value of S
Value of the total angular momentum J is
equal to |L-S| when the shell is less than half full
equal to L+S when shell is more than half full
equal J=S when the shell is half full (since application of first rule gives L=0)
Spin paramagnetism
odd # electrons OR even # if degen. electronic level is only partially filled
S=n/2, when there are n electrons in the given orbital and n≤2l+l
permanent mag. dipole moment μs = g0μBsqrt(S(S+1)) = μBsqrt(n(n+1))
μB = ℏe/2m
Orbital paramagnetism
arises from orbital angular momentum; electron in orbital with 1+ units of angualr momentum behaves like an electric current in circular wire = produces mag. moment; when all orbitals equally filled = orbital moments cancel out; may arise but be cancelled out by neighbouring atom/ion interaction; neglible contribution
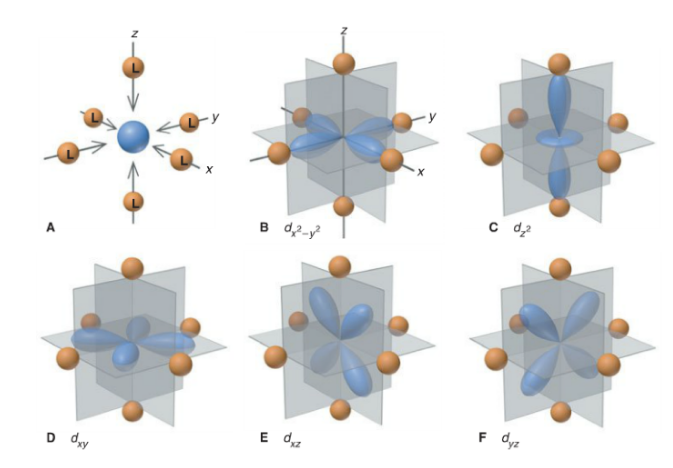
Transition metal complexes
metal surrounded by negative or neutral ligands
free transition element ion = 3d subshell has 5 degen. d orbitald
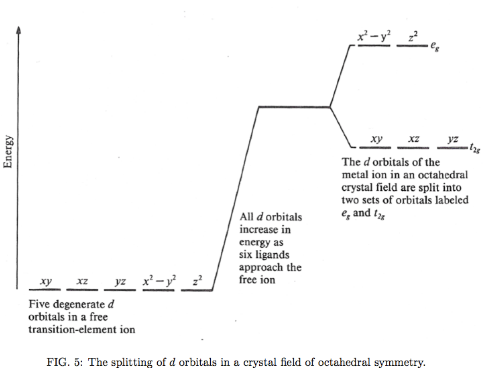
complex ion = ligands form electrostatic crystal field of high (octahedral) symmerty about the central metal ion; remove degeneracy
electronic balance
diamagnetic = repel = Δm>0
paramagnetic = attract = Δm<0