Edexcel Rate of reaction
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
Investigate the effects of changing the conditions of a reaction on the rates of chemical reactions by:
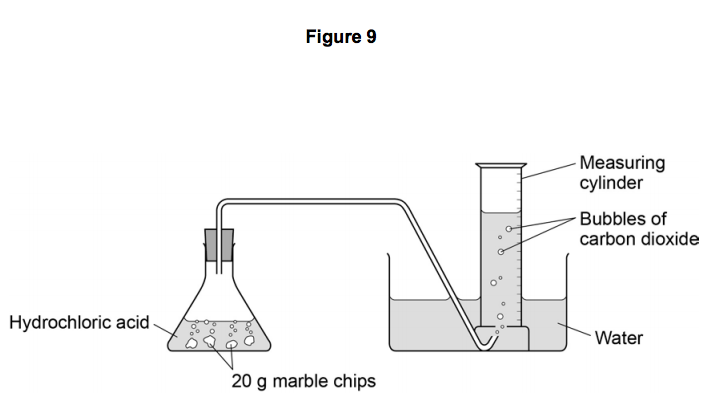
a measuring the production of a gas (in the reaction between hydrochloric acid and marble chips)
The finer the particles of solid, the larger the surface area of marble, so the faster the reaction.
- powdered chalk
- small chips
- large chips
The greater the mass of the marble chips also allows for a greater surface area. The extra surface area gives a faster reaction and there is more gas produced..
Investigate the effects of changing the conditions of a reaction on the rates of chemical reactions by:
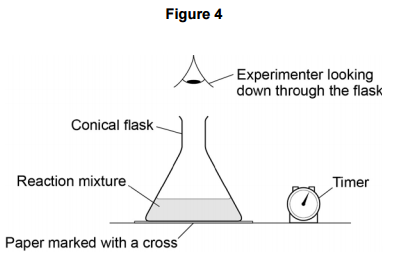
b observing a colour change (in the reaction between sodium thiosulfate and hydrochloric acid)
Place a flask over a black mark on a piece of paper which can be seen through the solution. Watch the black mark disappear through the cloudy, yellow sulphur and time how long it takes to go.
The hotter the temperature, the faster the colour change from cloudy to yellow, increasing the rate of the reaction.
Explain the effects on rates of reaction of changes in temperature in terms of frequency and/or energy of collisions between particles
The higher the temperature, the faster the particles move, resulting in more frequent collisions.
There is also an increase in the energy of the collisions, which means more successful collisions, increasing the rate of reaction.
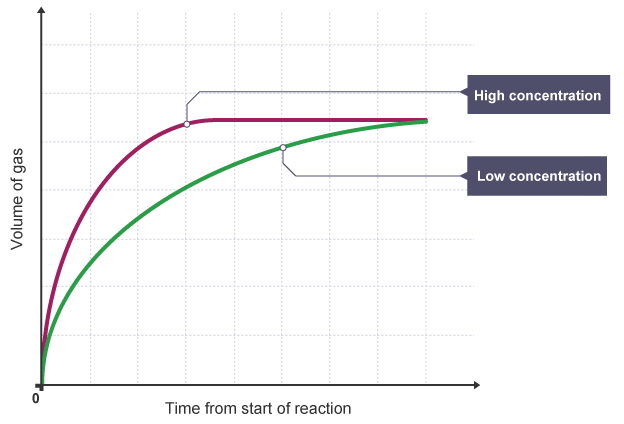
Explain the effects on rates of reaction of changes in concentration in terms of frequency and/or energy of collisions between particles
The higher the concentration, the more particles of reactant in a volume.
Collisions are more likely, so the reaction rate increases.
Explain the effects on rates of reaction of changes in surface area to volume ratio of a solid in terms of frequency and/or energy of collisions between particles
With a larger surface area:volume ratio, the particles around the solid have more area to work on, increasing the frequency of collisions will increase. So the reaction rate increases.
Explain the effects on rates of reaction of changes in pressure (on reactions involving gases) in terms of frequency and/or energy of collisions between particles
Increasing the pressure makes the particles more crowded.
The frequency of collisions will increase, so the rate of reaction will increase.
ACTIVATION ENERGY
Minimum energy needed for particles to react when they collide.
Explain how the addition of a catalyst increases the rate of a reaction in terms of activation energy
A catalyst decreases the activation energy needed for a reaction to occur, by providing an alternative activation pathway.
So, more particles have the minimum amount of energy needed for a reaction when they collide, increasing the rate of the reaction.
Rate of reaction
Describes how rapidly the reactants are consumed or the product is formed.
Collision Theory
Explains why different reactions occur at different rates and suggests ways to change the rate of a reaction.
Activation energy
The minimum amount of energy that particles must collide with to react.
Kinetic energy of the particles
The energy associated with the motion of particles.
Catalyst
Substances that speed up the rate of a reaction without being consumed in the reaction.
Surface area to volume ratio
The ratio that affects the rate of reaction in solids.
Pressure of reacting gases
A factor that influences the rate of reaction in gaseous systems.
Enzymes
Biological catalysts made from proteins that speed up reactions in living organisms.
Rate Graphs
Graphical representations used to measure the rate of reactions over time.
Factors affecting the rate of reaction
Nature of reactants
Temperature
Concentration of reactants
Surface area
Presence of a catalyst
factors affecting the collision of particles
-Number of particles per unit volume
-Frequency of collisions
-Activation energy
-Kinetic energy of the particles
Why will a chemical reaction occur?
When reactant particles collide with enough energy to react
Activation energy
The minimum amount of energy needed for a reaction to happen. Ea
Adding a catalyst
Increases the rate by providing an alternative reaction path with a lower activation energy
Increasing pressure
More particles in a smaller volume. More frequent collisions.
Increasing concentration
More particles in the same volume of liquid. More frequent collisions.
Increasing temperature
Gives particles more energy. Move faster, collide more frequently. More effective collisions (collisions with energy greater than Ea)
Increasing surface area
Exposes more particles. More frequent collisions.
How can you measure the rate of a reaction?
By experiment. Measure product produced or reactant used up over time.
What does a normal rate curve graph look like?
Steep curve in the beginning, becoming less steep, eventually flattening out when the reaction stops.