5. G-protein coupled Receptors (GPCRs) I
1/35
Earn XP
Description and Tags
complete
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
What are metabotropic receptors?
Metabotropic receptors are receptors indirectly linked to ion channels through signal transduction mechanisms, such as G proteins.
How many different families of receptor proteins are used to detect and respond to a myriad of chemical and physical stimuli, and which is the most diverse?
Cells use approximately 25 different families of receptor proteins to detect and respond to a myriad of chemical and physical stimuli.
GPCRs are the most diverse.
What are the key structural features of G-Protein Coupled Receptors (GPCRs)?
7 transmembrane α-helices (TMs).
TM3 centrally located next to binding pocket, crucial for ‘transduction’ of ligand binding
Other transmembrane helices (TMs) and the extracellular N-terminus also contribute to ligand binding.
N terminus at extracellular side (ligand binding)
C terminus at intracellular side (G-protein binding)
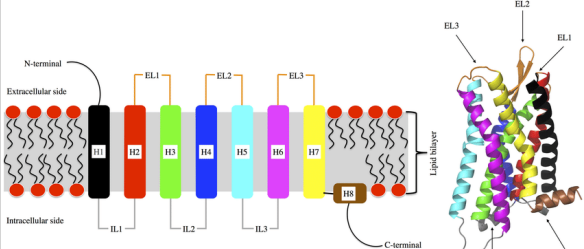
How are GPCR classes distinguished, and what is the significance of these differences?
GPCR classes are distinguished by the structural features of their extracellular domains, which define the ligand-binding site.
This diversity in structure is directly linked to the wide range of stimuli that GPCRs can detect.
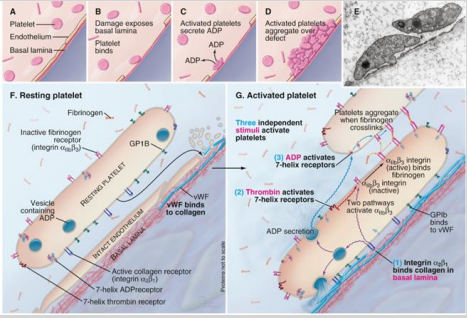
How are protease-activated receptors (PAR) in platelets activated, and how do they contribute to platelet activation?
Activated by the cleavage of the N-terminal part of the receptor, which then acts as a tethered ligand—part of the receptor itself functions as the agonist.
Platelet activation requires three independent stimuli to work together, ensuring a coordinated response.
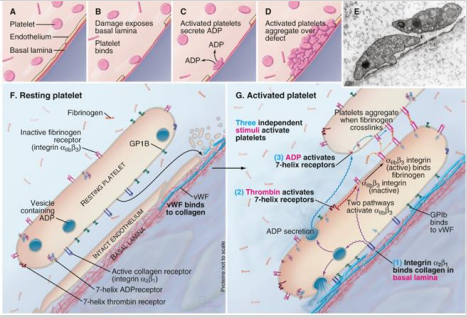
What are the 3 independent stimuli activating platelets?
ADP
Thrombin
Basal Lamina
What are G-proteins?
Guanine nucleotide-binding proteins – belong to the GTPase family.
Act as molecular switches inside cell to transmit signals from extracellular stimuli.
Regulated by ability to bind & hydrolyse GTP (‘on’) to GDP (‘off’).
Exist as heterotrimeric complexes made up of ⍺, β, and 𝛾 subunits.
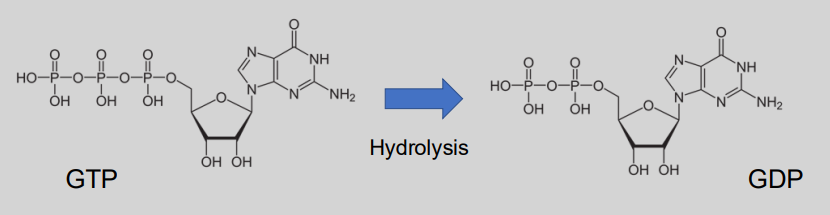
What is the basic mechanism of GPCRs? (3)
A. resting (3 inactive components)
B. Activation of ligand binding and exchange of GDP for GTP
C. 3 separate active components
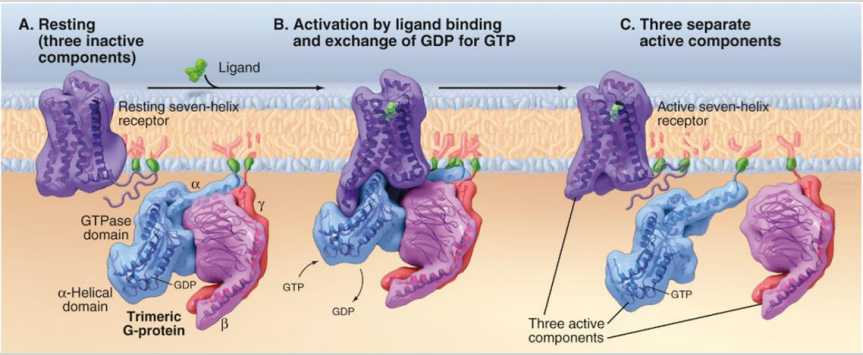
What is the mechanism of action for G proteins in signal transduction? (4)
In the inactive state, GDP is bound to the alpha (⍺) subunit of the G protein.
Ligand binding to the receptor causes a conformational change, activating the G protein.
GDP is released from the ⍺ subunit, which then binds GTP, activating the G protein.
The ⍺ subunit separates from other subunits and binds to a target protein in the membrane, triggering a cellular response.
How is G-protein signaling controlled?
G-proteins act as timers
The duration of signalling by the activated G protein is regulated by the rate of GDP hydrolysis by the ⍺ subunit.
RGS (Regulators of G-protein Signalling) proteins stimulate GTPase activity in the ⍺ subunit, speeding up the conversion of GTP to GDP, thereby terminating the signal.
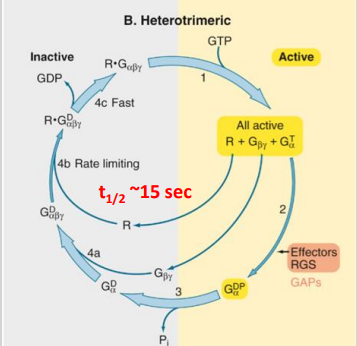
How many families of G-proteins are there and how do they contribute to signal diversity?
6 different families of G-proteins.
Various combinations of their ⍺, β, and γ subunits allow for a wide range of cellular responses.
Each family can activate different downstream pathways, increasing the diversity of signals triggered by GPCR activation.
How does the Golfα subunit contribute to the specificity of smell detection in certain cells?
The Golfα subunit is specifically expressed in olfactory cells. This cell-type-specific expression of particular receptors and signal transduction molecules, like Golfα, allows cells to respond specifically to odorant molecules. Even though these cells use similar GPCR signalling mechanisms as other cell types, the specific subunit and receptor combination enables unique responses to smell stimuli.
How is the effector in G-protein signalling determined, and what are common types of effectors?
The effector in G-protein signalling is determined by the class of the Gα subunit. Effectors can include:
Enzymes that produce second messengers (e.g., adenylyl cyclase or phospholipase C)
Ion channels, which can be regulated directly by βγ subunits or indirectly through second messengers and their effectors.
What are the characteristics of ion channels that are directly activated by G-proteins?
They share a similar mechanism with ligand-gated channels.
They are slow to open or close compared to other channels.
Once activated, they tend to stay open or closed for longer periods (minutes rather than milliseconds).
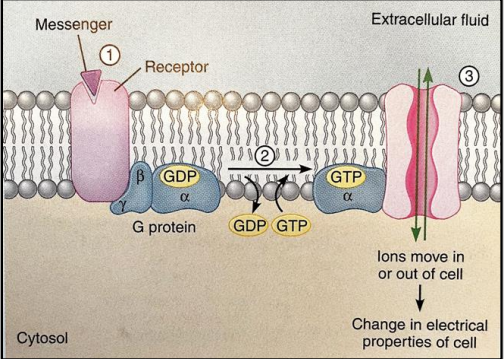
What role do activated G proteins play in relation to second messengers, and what are some examples of second messengers?
Activated G proteins regulate the activities of enzymes that control the levels of second messengers.
Second messengers are small molecules that carry signals inside cells and include:
Hydrophobic lipids confined to the membrane where they are generated.
Small soluble molecules that diffuse through the cytoplasm (e.g., cAMP, cGMP).
Calcium ions (Ca²⁺).
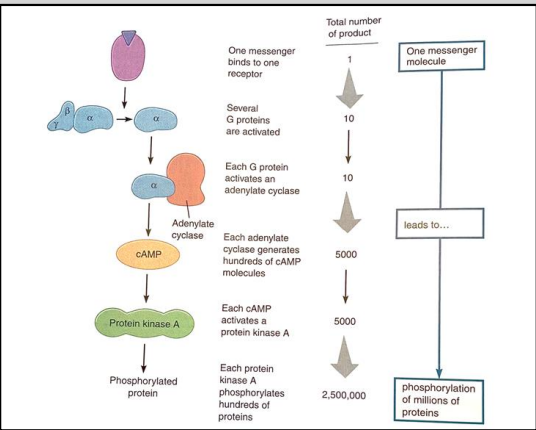
Why are second messenger systems important in cellular signalling?
A single ligand binding to a single GPCR can lead to the phosphorylation (activation) of millions of proteins.
This amplification effect allows cells to respond efficiently and rapidly to external signals, facilitating complex cellular responses and enabling coordination of various cellular processes.
What bacterium is responsible for cholera?
Vibrio cholera.
Which G-protein is involved in the mechanism of cholera toxin?
Gs α subunit (stimulatory).
What second messenger is activated by cholera toxin?
Cholera toxin activates adenylate cyclase, leading to increased levels of cAMP (through catalysis).
What is the effect of cAMP activation on protein kinases in cells affected by cholera toxin?
Activation of cAMP leads to the activation of protein kinases
What happens to ion secretion in cells due to cholera toxin?
Increase in Cl⁻ ion secretion
Increase in Na⁺ ion secretion, along with H2O.
What is the physiological effect of cholera toxin on the small intestine?
An excess of fluid and electrolytes in the lumen of the small intestine.
What are the primary symptoms of cholera?
The primary symptoms of cholera are diarrhoea and extreme dehydration.
What bacterium is responsible for whooping cough?
Bordetella pertussis.
Which G-protein is involved in the mechanism of whooping cough?
Gi α subunit (inhibitory).
What second messenger is activated in the case of whooping cough?
The second messenger activated is cAMP due to the inactivation of the inhibitory G protein.
What effect does increased cAMP have on cells during whooping cough?
The effect is too much intracellular cAMP.
What is the physiological effect of whooping cough on the respiratory system?
It leads to erosion of the respiratory epithelium and the discharge of large quantities of mucus-containing fluid.
What is a primary symptom of whooping cough?
Coughing fits.
What structural changes can result from mutations in GPCRs?
Changes in the receptor's transmembrane helices
Affect the ligand binding pocket
Alter the extracellular and intracellular loops, impacting receptor stability and function.
What are some diseases associated with mutations in GPCRs?
Hypoparathyroidism, thyroid cancer, colour blindness, e.t.c.
What percentage of uveal melanoma cases have mutations in the Gq α subunit (GNAQ and GNA11)?
Over 90% of uveal melanoma cases have mutations in the Gq α subunit.
What effect do GNAQ and GNA11 mutations have on GTP hydrolysis?
These mutations block GTP hydrolysis, causing the Gq α subunits to be constantly active.
What is the consequence of GNAQ and GNA11 mutations leading to constantly active Gq α subunits?
They result in permanent signal transmission, leading to constitutive activation of growth pathways.
When do GNAQ and GNA11 mutations typically occur in uveal melanoma?
These mutations are thought to occur early in tumour development.
Can GNAQ and GNA11 mutations in uveal melanoma be targeted for therapy?
Currently, they cannot be targeted for therapy, but they can be useful for diagnosis.