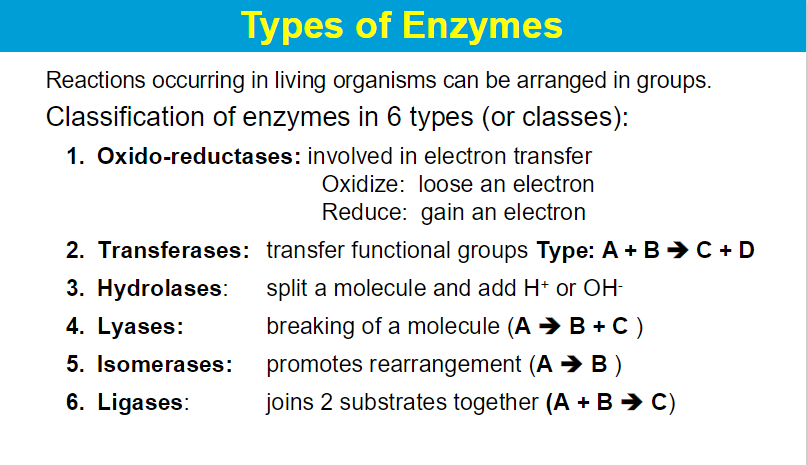
Enzyme reactions and kinetics
**Living organisms must be able to carry out reactions which are very
thermodynamically unfavorable (at body’s temperature)
Enzymes are biological catalysts.
increase reaction rates without being itself being affected or consumed;
Most enzymes are globular proteins but can be also RNA;
Effective in small amounts;
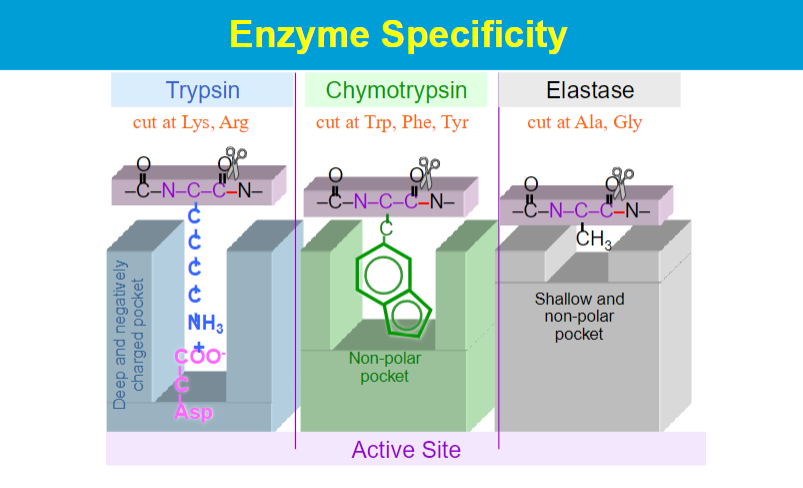
Highly specific (1 enzyme for 1 reaction);
Usually unstable/short-lived
Some RNA (ribozymes and ribosomal RNA) also catalyze reactions
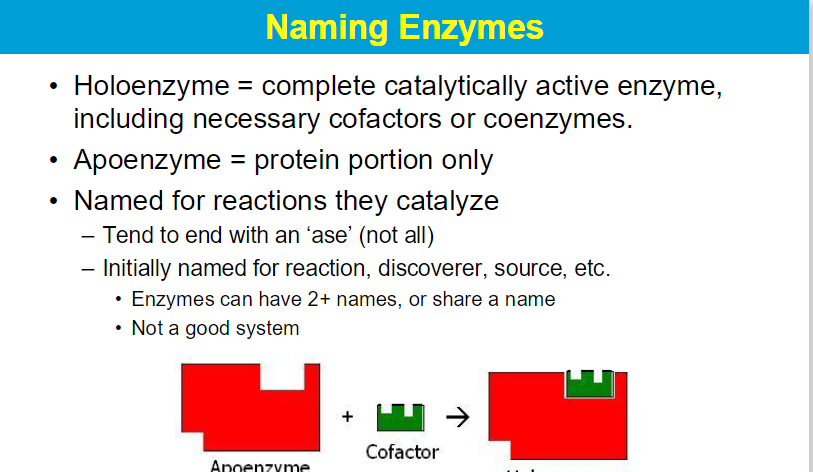
Enzymes are identified through their “enzyme classification” (EC) number. This is a code composed of 4 consecutive numbers assigned to each enzyme on the basis of the type of reaction that it catalyzes.
The first EC number defines the class to which each enzyme belongs. There are six classes of enzymes.
The subsequent three numbers define increasingly smaller and more specific subclasses.

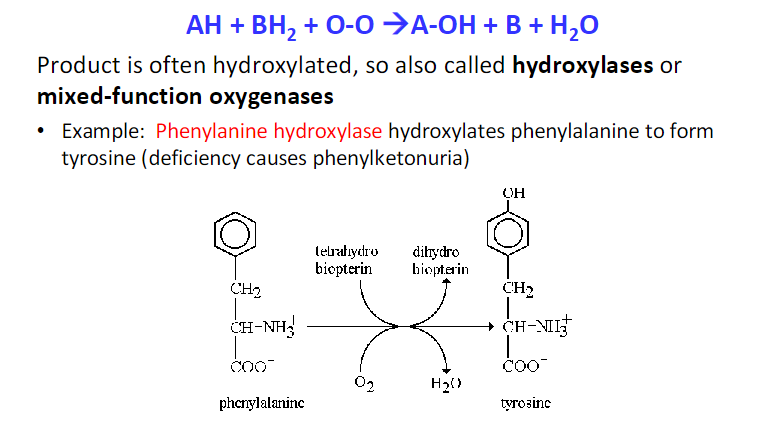
Cytochrome P450 enzymes (CYPs) are a large superfamily of heme-containing monooxygenases; catalyze many oxidation reations
they do so by incorporating one atom of oxygen into the substrate (RH) while reducing the other oxygen atom to water.
This reaction typically converts nonpolar substrates into hydroxylated products (ROH), increasing their polarity and facilitating their excretion.
Hydroxylation usually inactivates the substrate, rendering it less active pharmacologically and making it more water-soluble. This modification is crucial for drug metabolism, as it enhances the elimination of drugs from the body.
If two drugs (or alcohol and a drug) use the same P450, they will compete, and levels of the drug or alcohol will not be cleared as quickly
Dioxygenases are a type of oxidoreductase enzyme that catalyze the incorporation of both atoms of molecular oxygen (O₂) into substrates.
often metalloproteins (heme), typically containing iron (Fe) or manganese (Mn) ions in their active sites.
Many dioxygenases are involved in the oxidation of aromatic compounds, often leading to the cleavage of aromatic rings
ex.
Cyclooxygenases (COX) enzymes catalyze the conversion of arachidonic acid into prostaglandins and thromboxanes, which are important mediators of inflammation, pain, and fever.
NSAID Target: Nonsteroidal anti-inflammatory drugs (NSAIDs), such as aspirin and ibuprofen, inhibit COX enzymes to reduce pain and inflammation by decreasing prostaglandin production.
also converts catechold to ketones
Catechol 1,2-dioxygenase,
Tryptophan 2,3-dioxygenase
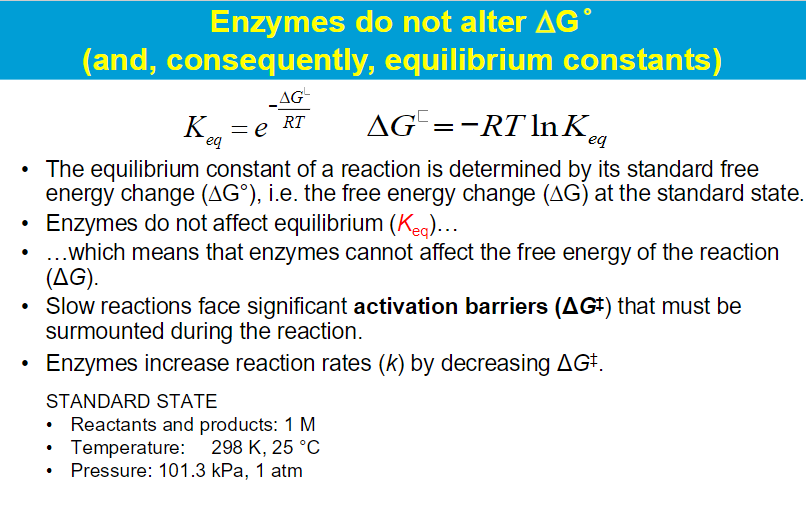
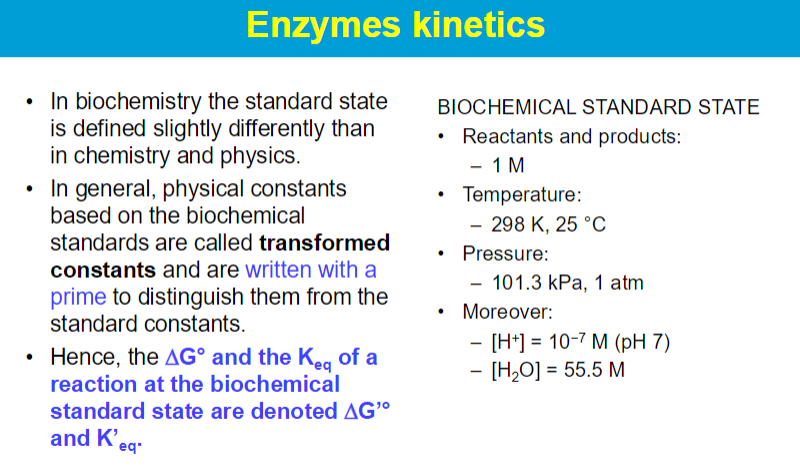
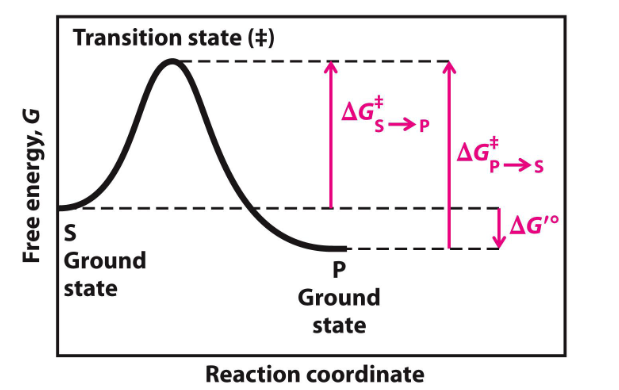
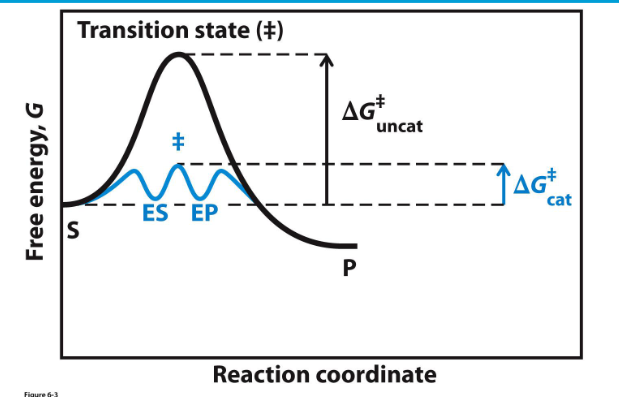
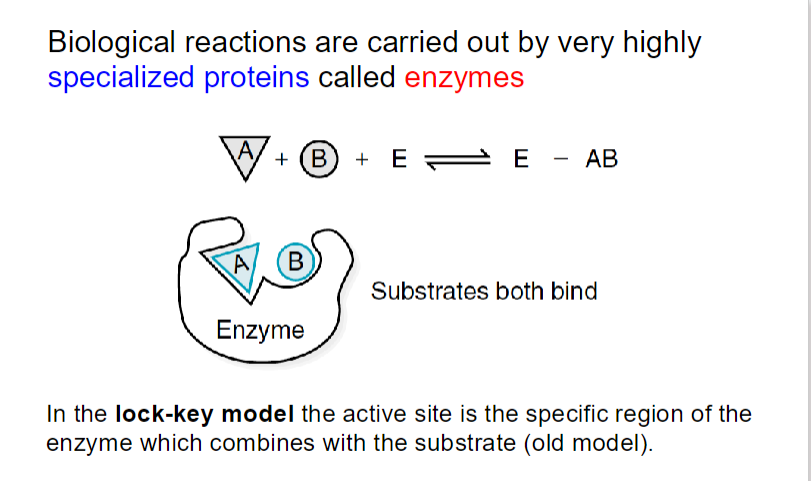
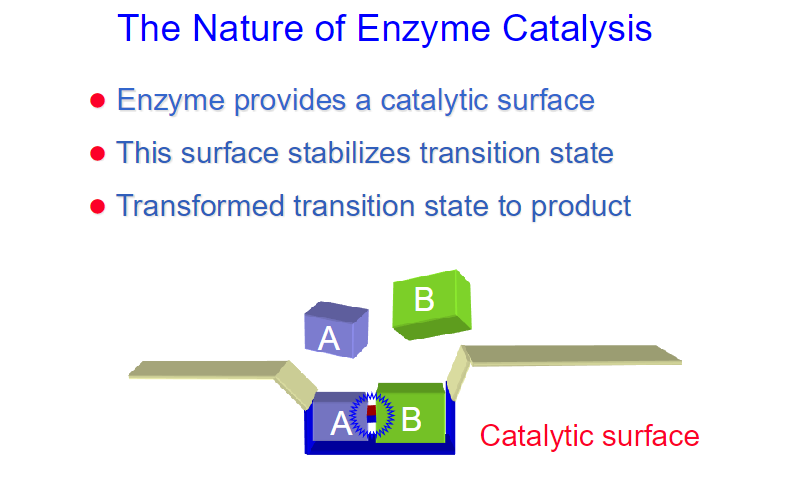
**Enzymes have a higher binding affinity for the transition state than for the substrate. This stronger interaction reduces the activation energy required for the reaction to proceed. By stabilizing the transition state, enzymes effectively lower the energy barrier that must be overcome for the reaction to occur.
Enzymes bring substrates together in close proximity, aligning them in the correct orientation to promote a reaction.
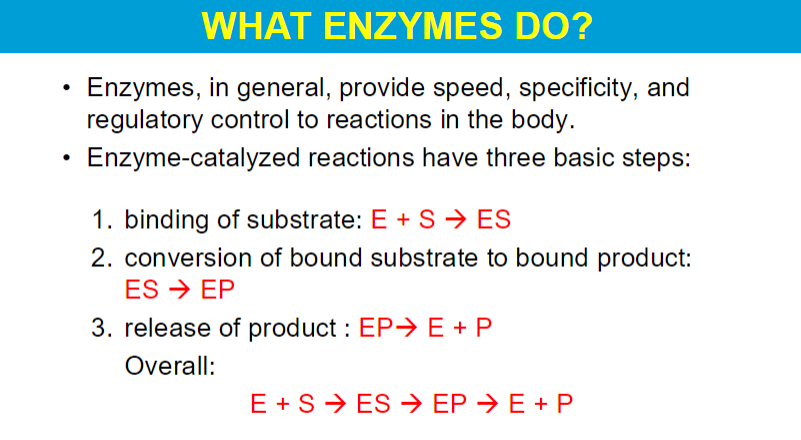
When substrates bind to an enzyme, the enzyme forms a stable enzyme-substrate (ES) complex.
The formation of the ES complex reduces the degrees of freedom of the substrates, resulting in an increase in order (a decrease in entropy). This organization can be viewed as an "entropy cost" because the substrates become more constrained compared to their free states.
Once the substrates are organized into the ES complex, the conversion of this complex into the transition state does not require further significant changes in orientation or proximity. This process is entropically neutral because the rigid ES complex has already reduced the entropy
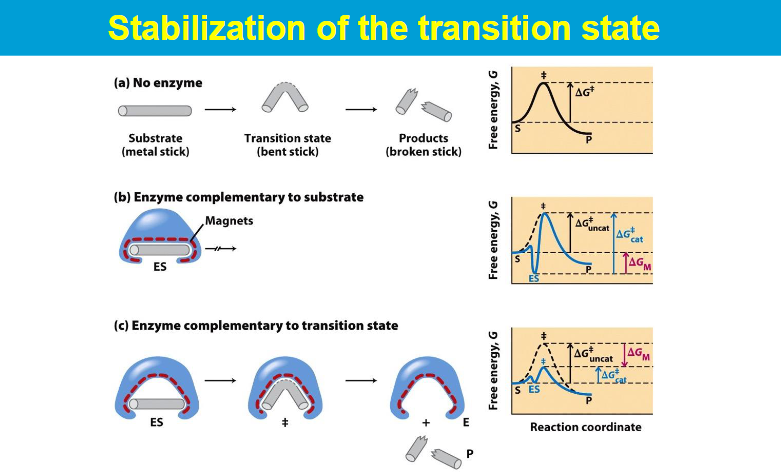
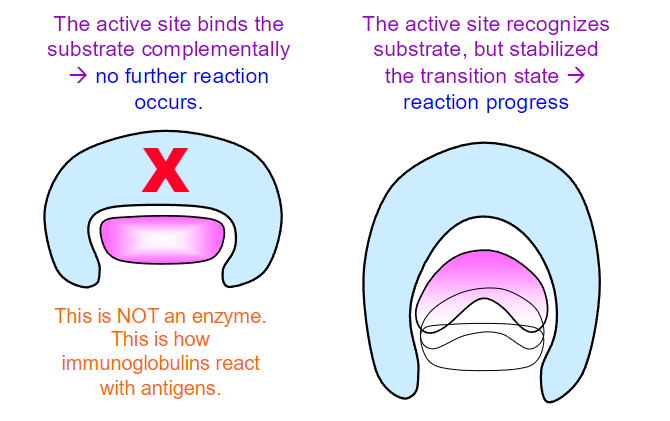
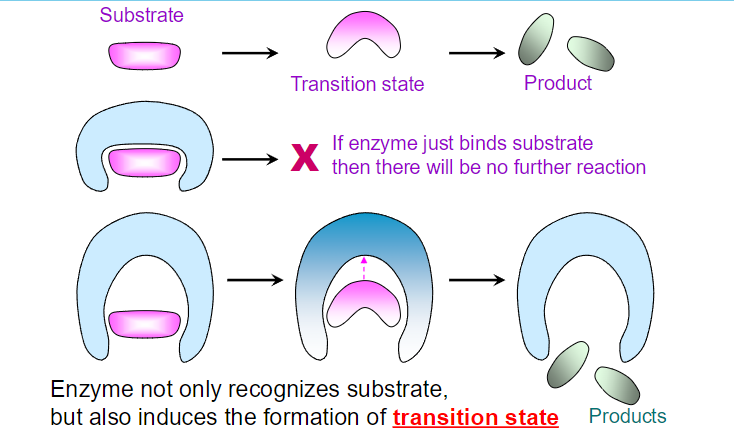
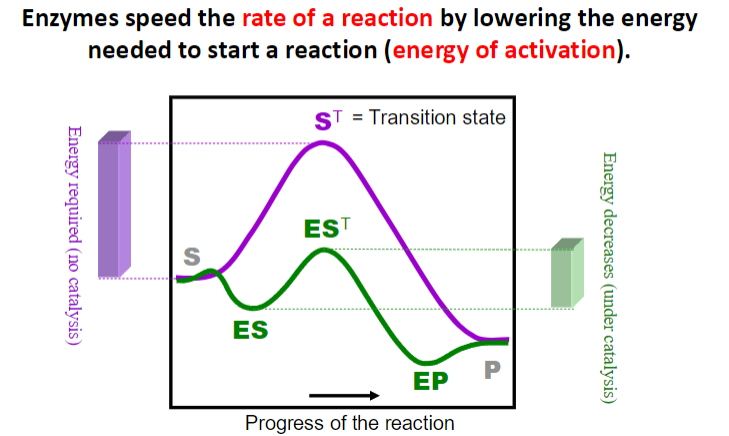
while the binding energy of the enzyme-substrate complex is crucial for stabilizing the transition state, enzymes often employ various catalytic strategies to further enhance reaction rates.
Acid-Base Catalysis: This mechanism involves the transfer of protons (H⁺ ions) to and from substrates, which can stabilize charged intermediates and facilitate the breaking or forming of bonds.
Covalent Catalysis : This mechanism involves the formation of a transient covalent bond between the enzyme and the substrate, leading to a change in the reaction pathway.
Metal Ion Catalysis: This mechanism involves the participation of metal ions in enzymatic reactions, which can facilitate various processes, including electron transfer and stabilization of charged intermediates.
Once the enzyme-substrate complex is formed, the positioning of functional groups within the active site is critical:
These groups are arranged to facilitate bond formation or cleavage effectively.
The rigid structure of the enzyme allows for precise interactions with the substrate, enhancing the likelihood of the reaction occurring.
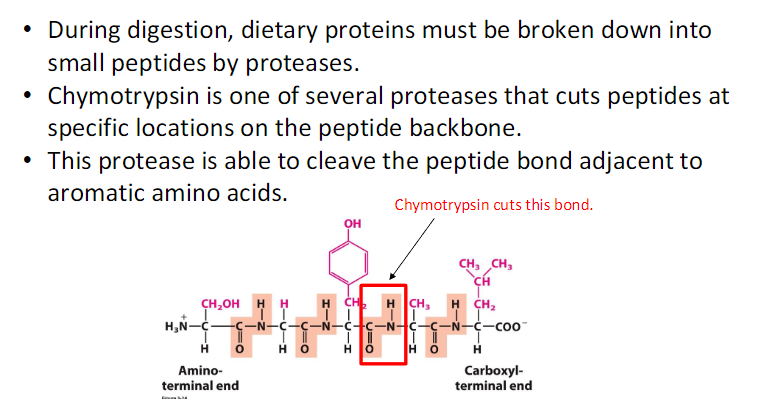
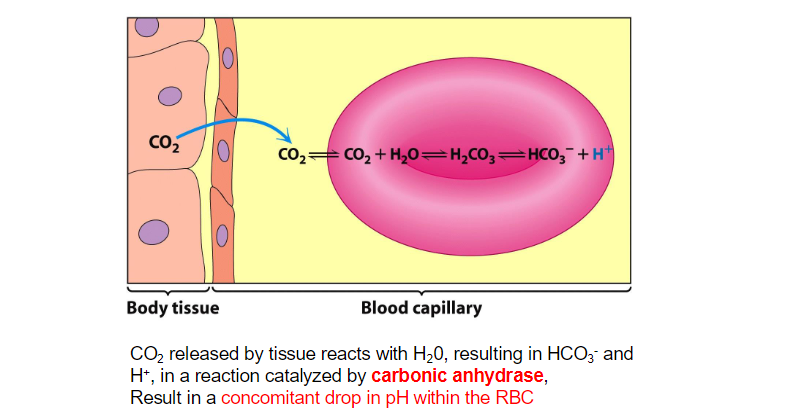
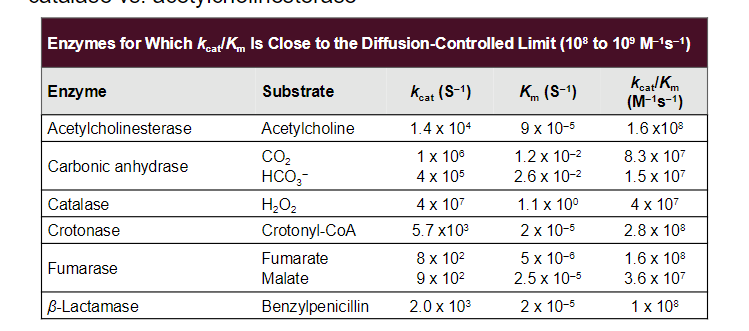
Carbonic Anhydrase (CA) is an incredibly efficient enzyme;
Facilitates the hydration of CO2 near metabolizing tissues, or the (reverse) reaction of dehydrating bicarbonate ion near the lungs to mediate the exhalation of CO
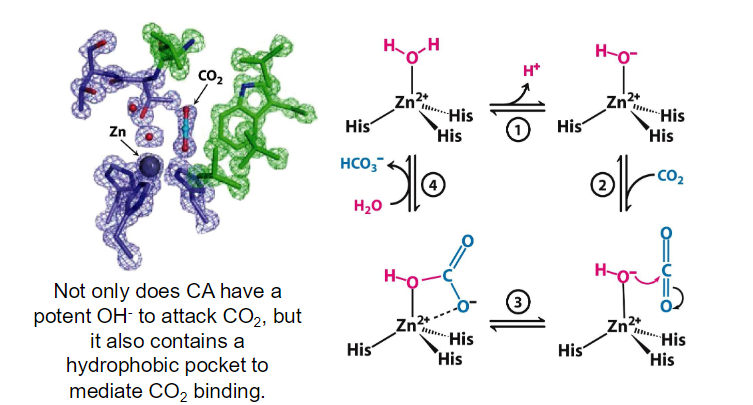
metal ion-mediated catalysis
Carbonic anhydrase contains a zinc ion at its active site. The Zn²⁺ ion is coordinated by three histidine residues and a water molecule
The coordination of a water molecule to the zinc ion facilitates its deprotonation. The zinc ion stabilizes the negative charge that develops on the hydroxide ion (OH⁻) after the removal of a proton (H⁺) from water. This results in a highly reactive hydroxide ion, which is now capable of attacking CO₂.
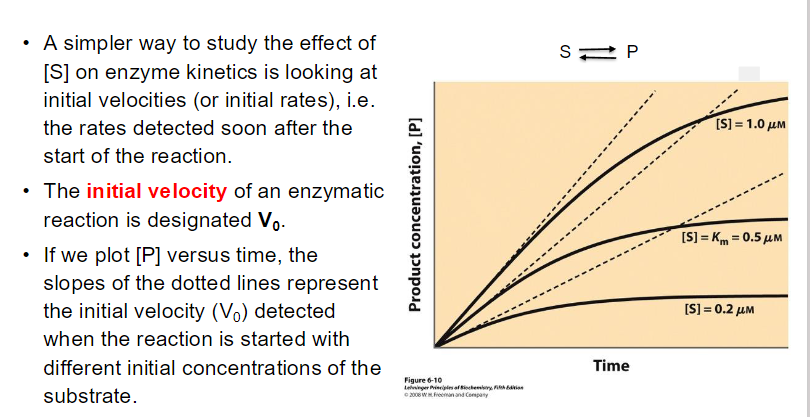
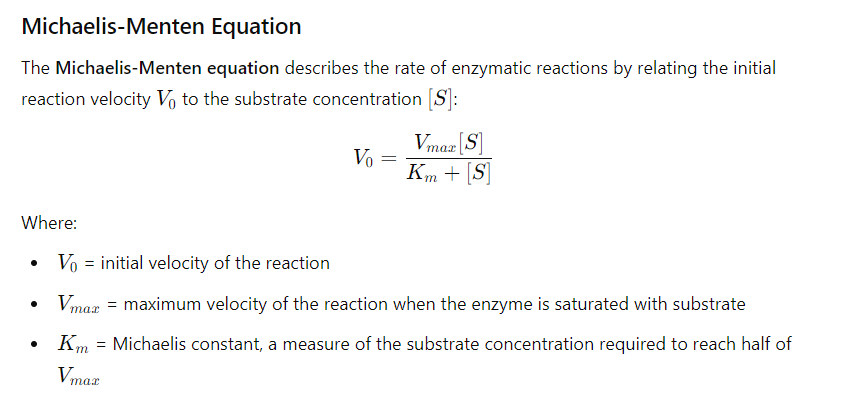
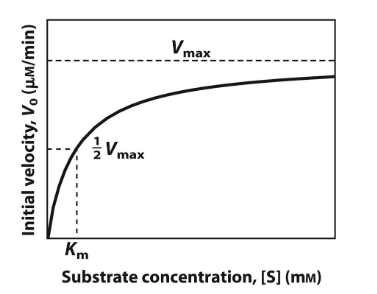
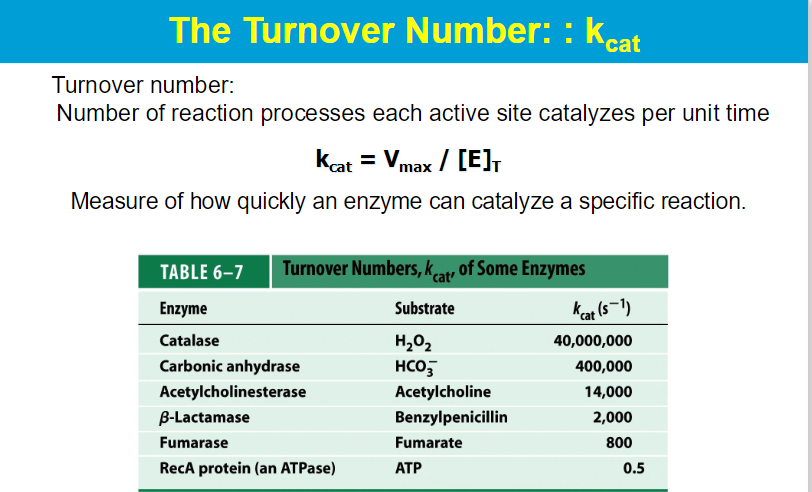
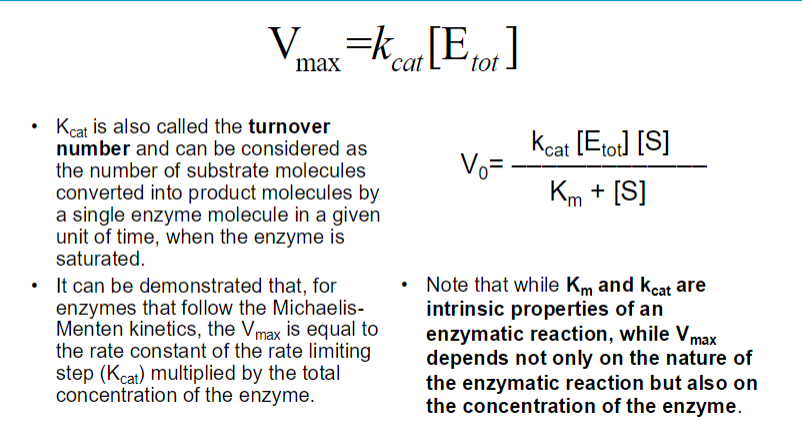
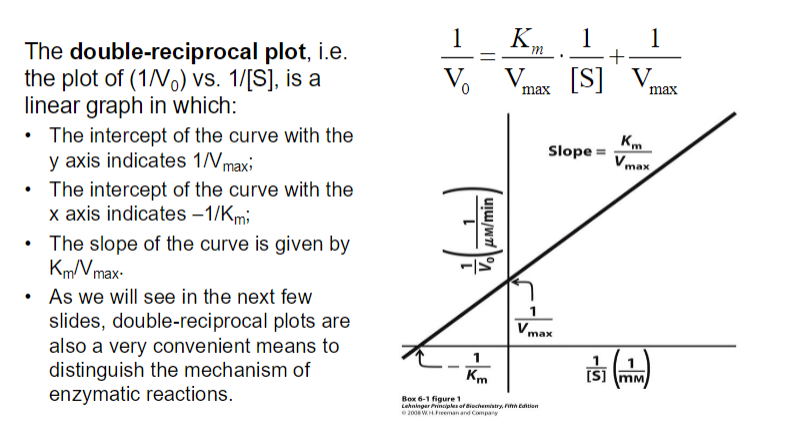
small Km : Indicates tight binding between the enzyme and substrate, meaning that a low concentration of substrate is needed to achieve half of Vmax.
This suggests that the enzyme is efficient at low substrate concentrations.
High Km : Suggests weak binding, meaning that a higher concentration of substrate is needed to achieve half of Vmax
This indicates that the enzyme is less efficient at lower substrate concentrations.
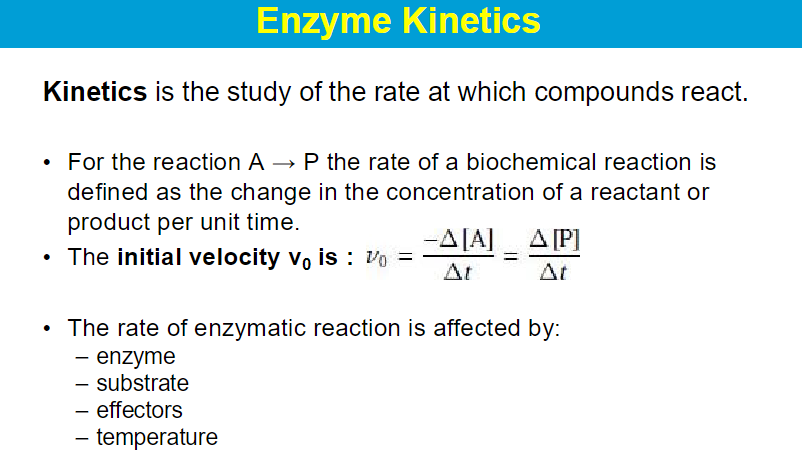
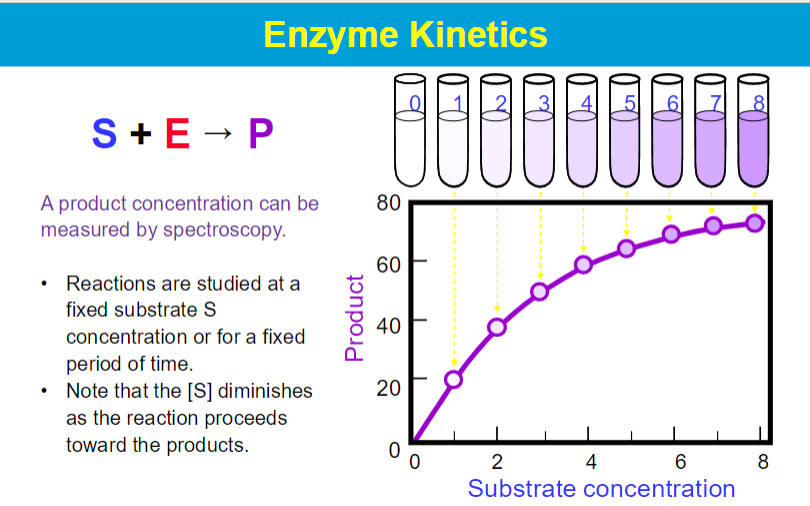
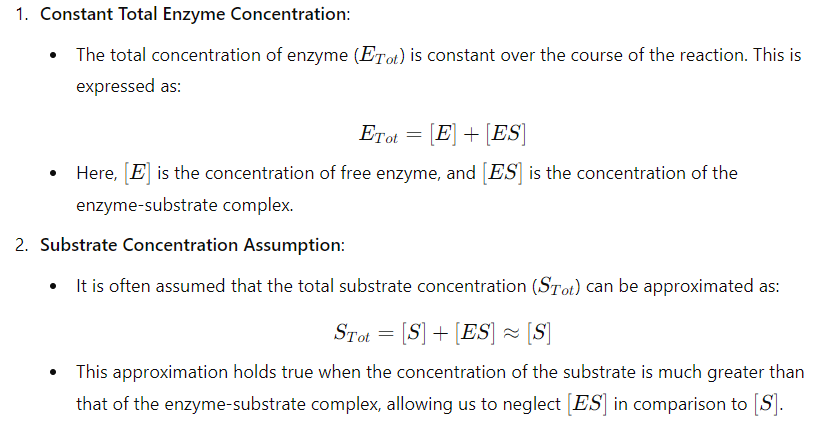
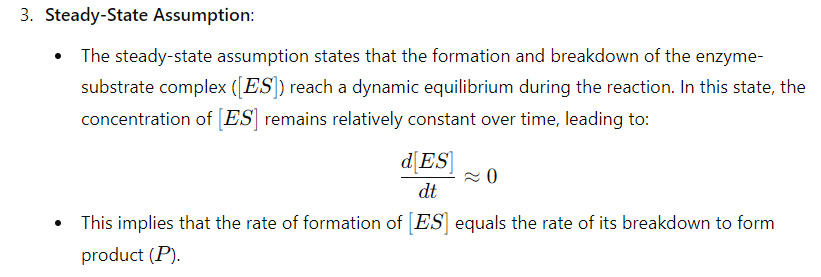
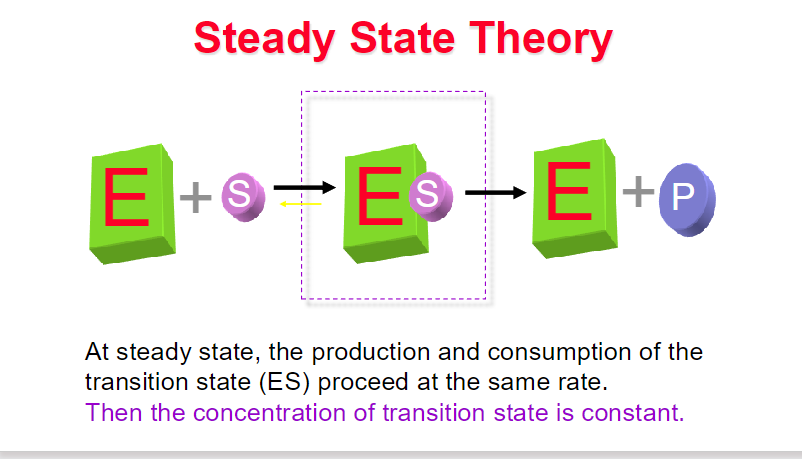
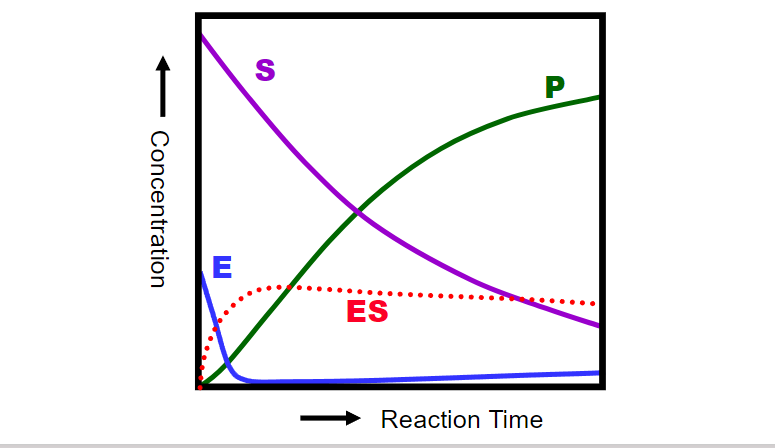
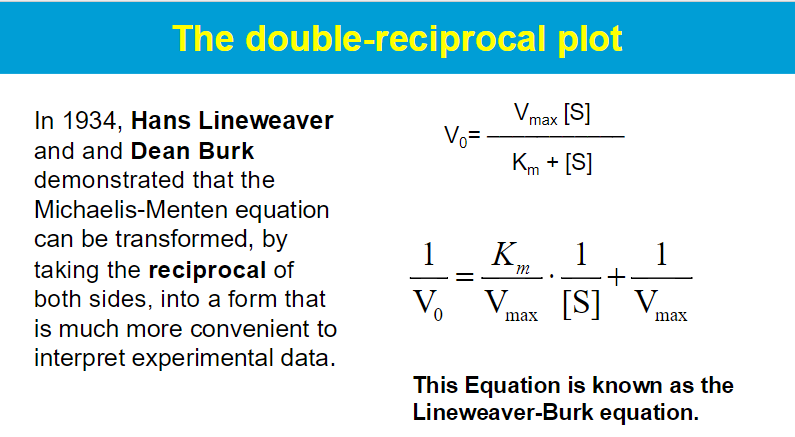
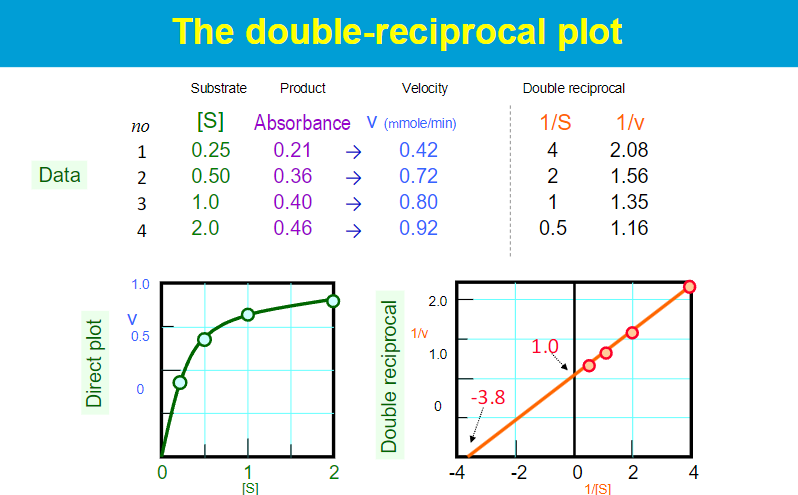
key assumptions of the Michaelis-Menten kinetic mode


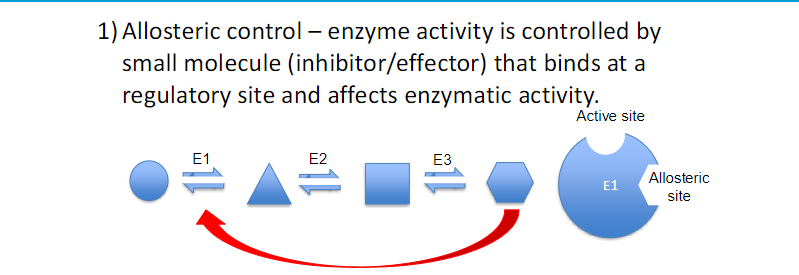
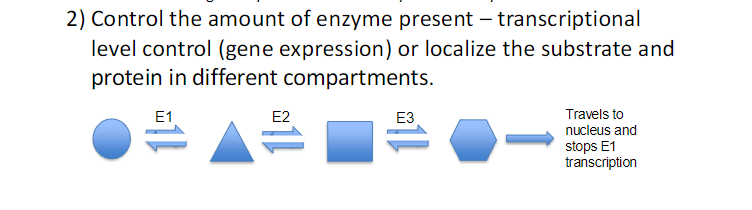
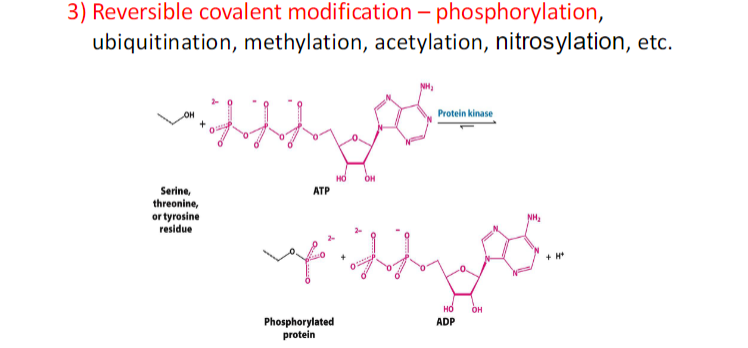
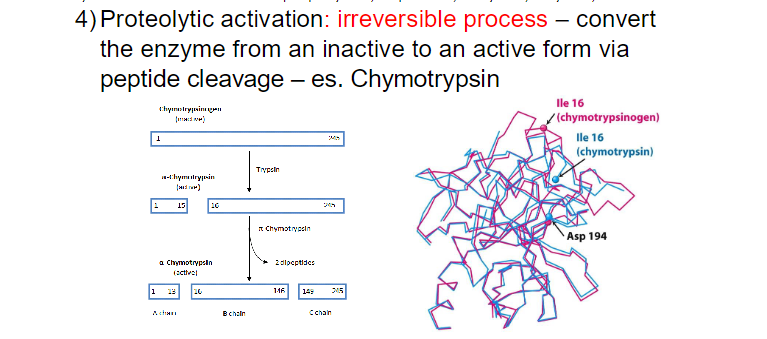
ENZYME REGULATION ( 4 ways)