Maintenance of Anaesthesia
1/86
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
87 Terms
What are the two different types of inhalation agents?
Vapours and gases
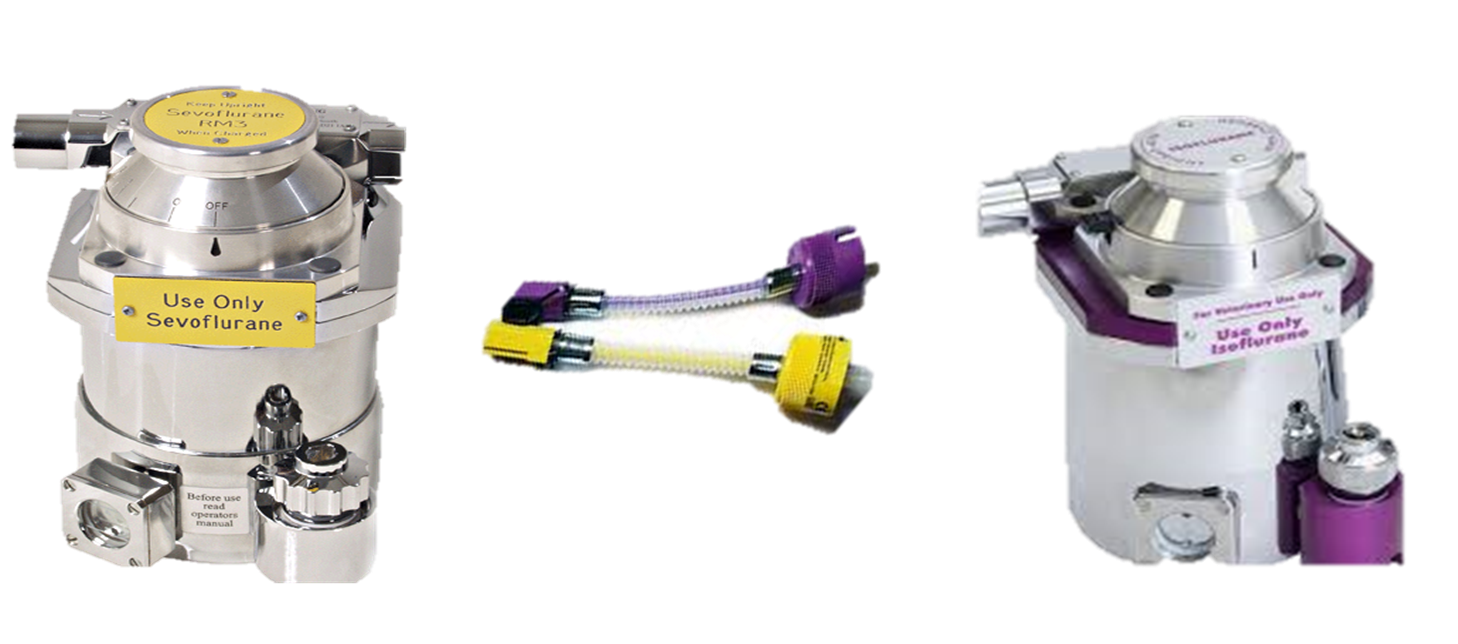
what colour is iso and sevo
sevo yellow
iso purple
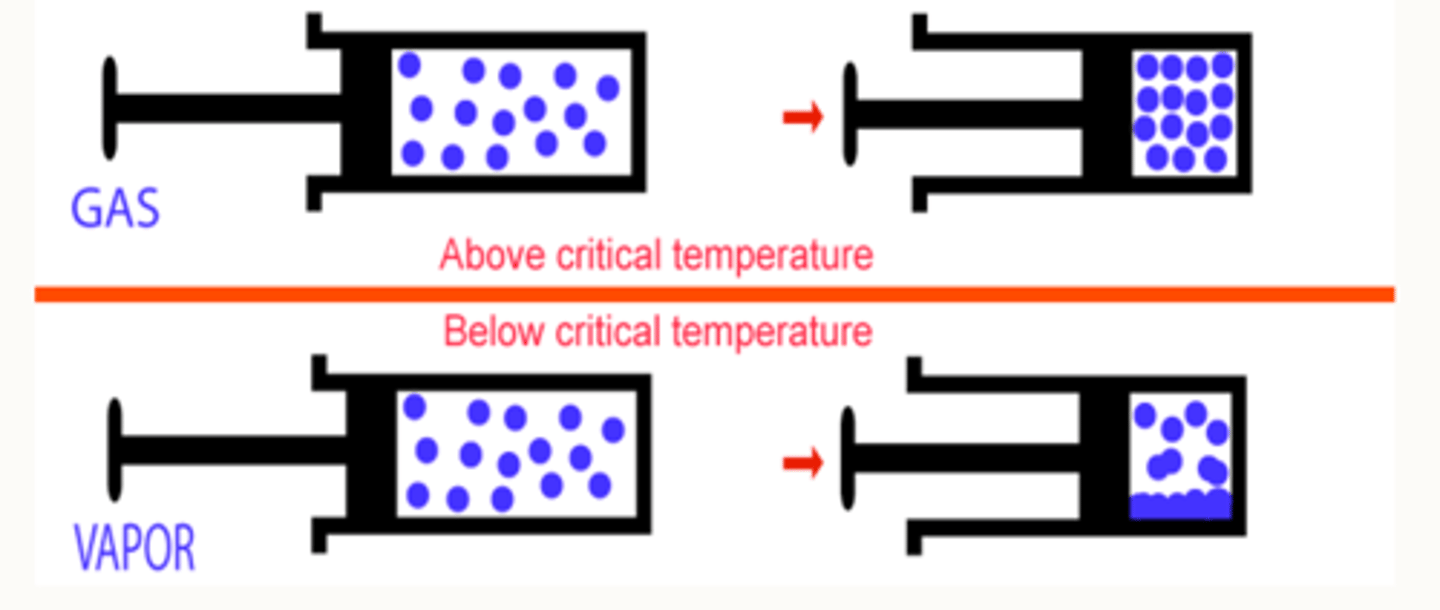
What is critical temperature?
The critical temperature is the temperature above which a gas cannot be liquefied by increasing pressure alone (cooling is also required).
what happens if a substance is above its critical temp
The substance cannot be turned into a liquid, no matter how much pressure is applied, it will always remain as a gas.
what happens if a substance is below its critical temp
The substance can be turned into a liquid by increasing pressure, this is known as a vapour
are iso and sevo vapours or gas
vapours
What is the difference between vapours and gases?
Gas exists only as a gas at room temp (e.g. oxygen). Vapour comes from a liquid and can exist as liquid + gas at room temp (e.g. volatile anaesthetics)
Volatile anaesthetics are liquids that are vapourised into a carrier gas
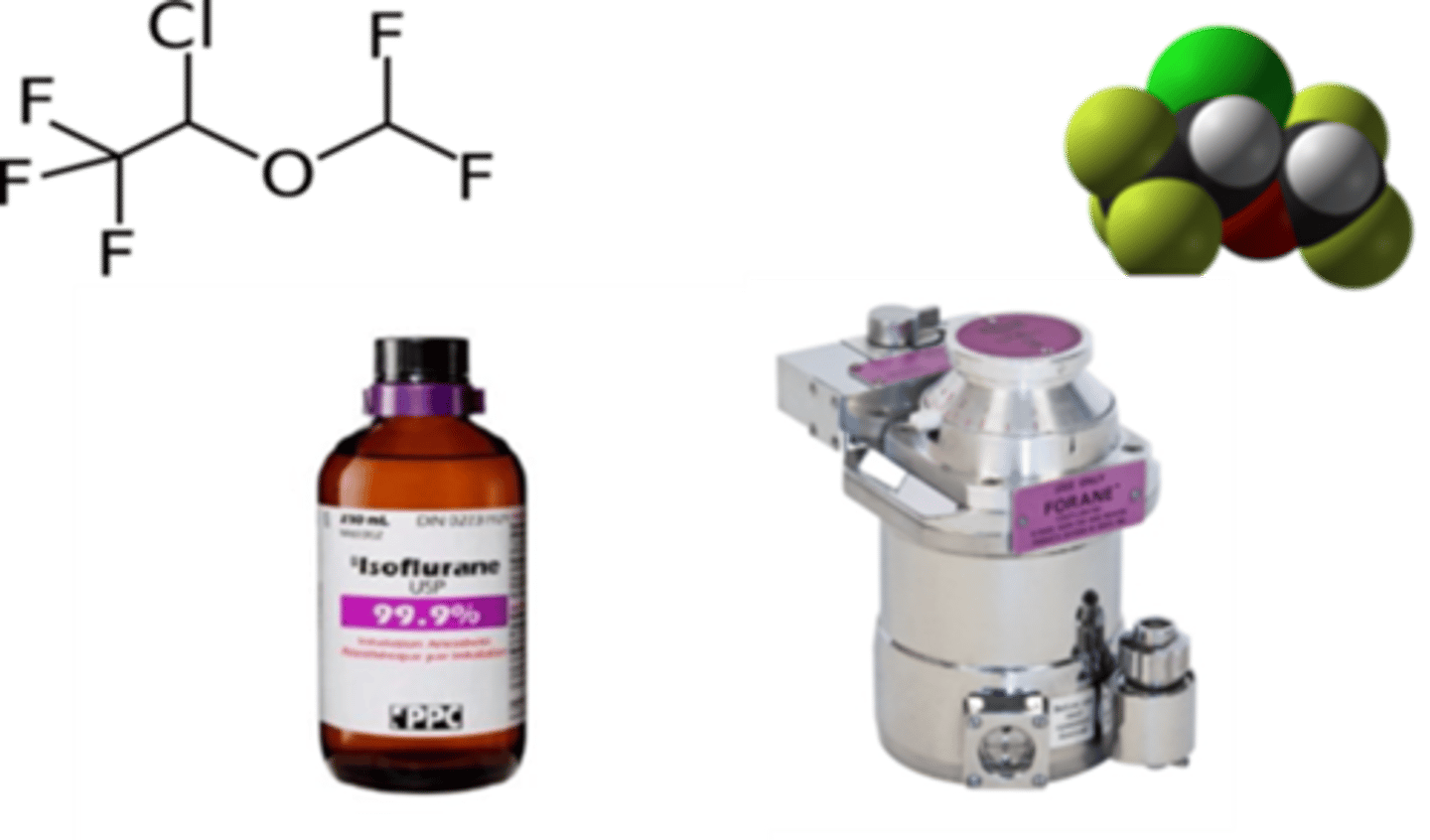
how are iso and sevo delivered as a vapour
Volatile anaesthetic agents (e.g., isoflurane, sevoflurane) are below their critical temperature at room temperature, so they exist as liquids and produce vapour.
Because these agents are liquids at room temperature, they must be vaporised into a carrier gas before they can be delivered to the patient.
This is why vaporisers are required to deliver volatile anaesthetic agents safely and accurately during anaesthesia.
what does a vapouriser do
converts the liquid inhalation agent to vapour
List inhalation agents that are vapours in their gaseous state
Isoflurane - purple
Sevoflurane - yellow
Halothane = old
Desflurane = need electricity, not common

Since Isoflurane and Sevoflurane are vapours in their gaseous state, what are they supplied as?
Liquids in pressurised cylinders (high pressure keeps them in liquid state and then when heated below critical temp they enter supercritical state between liquid and gaseous vapour molecules which escape to be inhaled by the patient)

What is Isoflurane commonly used for?
To induce and maintain anaesthesia in various animals. Inhalant anaesthetic that enhances GABA and glycine receptor activity while inhibiting excitatory neurotransmission.
Licensed for use in: Dogs, cats, horses, chinchillas, ferrets, gerbils, guinea pigs, hamsters, mice, rats, ornamental birds, and reptiles.
Why is isoflurane better than halothane?
- Isoflurane has lower solubility than Halothane (so iso has faster induction).
- Isoflurane is generally considered safer in terms of maintaining cardiovascular stability (dose-dependent) due to its minimal myocardial depression and better preservation of cardiac output, though it can cause vasodilation and reflex tachycardia (to compensate for lowered BP from vasodilation). In contrast, halothane has more pronounced myocardial depressant effects and a higher arrhythmogenic potential, making it less favourable in patients with pre-existing cardiovascular issues.
- Studies have shown significantly fewer anaesthetic deaths in cats, dogs, and horses with Isoflurane compared to Halothane.
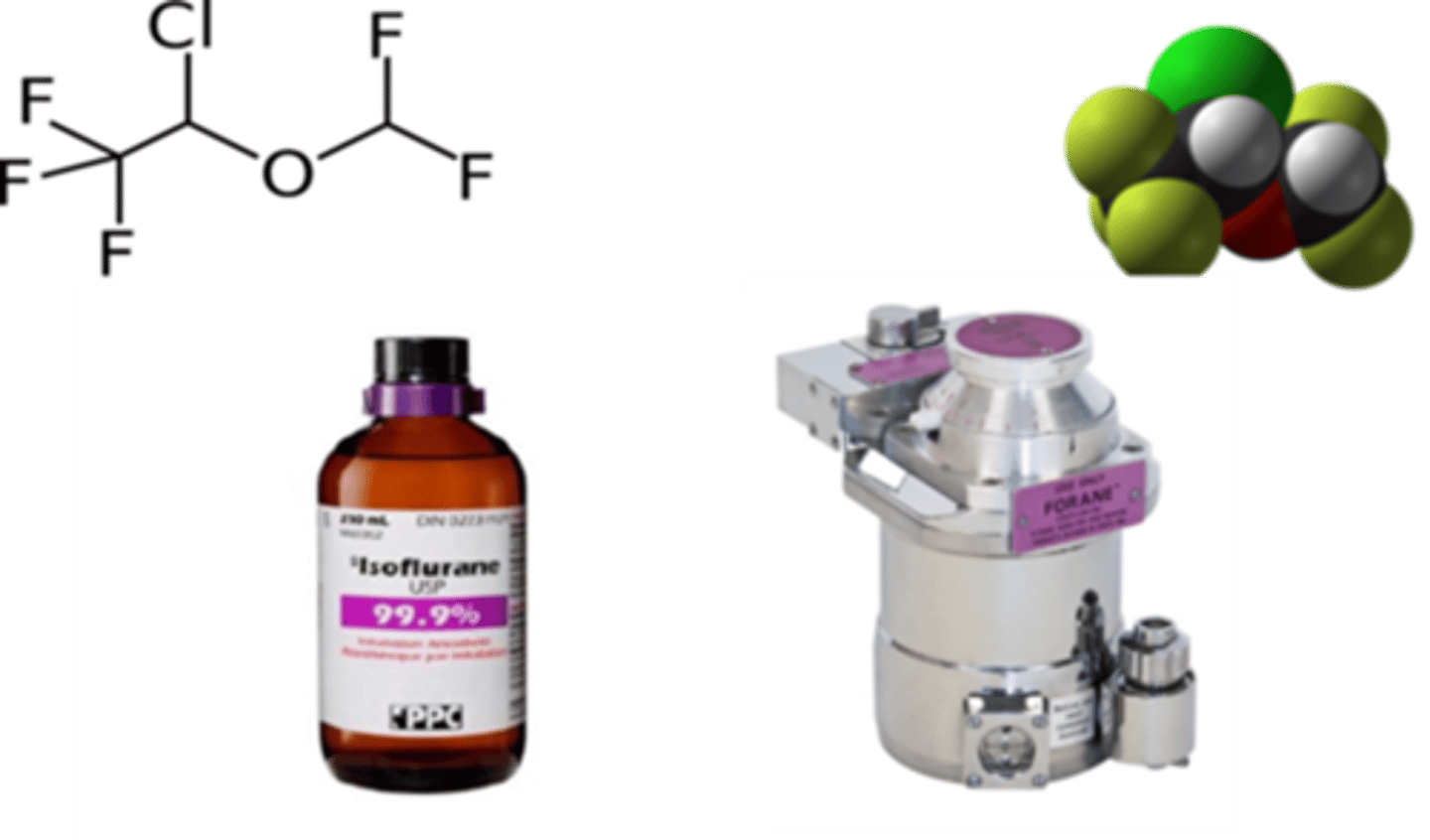
isoflurane mac values dog, cat, horse, foal, rabbit
dog - 1.28%
cat - 1.63 %
horse - 1.3%
foal - 0.9%
rabbit - 2.05%
What is Sevoflurane used for?
To induce and maintain anaesthesia in various animals, but also helpful for recovery. Inhalant anaesthetic that enhances GABA and glycine receptor activity while inhibiting excitatory neurotransmission.

sevoflurane key features
lower blood-gas solubility than isoflurane
faster depth changes and recovery
dose dependent cardiovascular depression
more expensive
sevoflurane mac values
dog 2.2%
cat 2.58%
horse 2.3%
rabbit 3.7%
iso key features
Widely used modern agent
Moderate blood-gas solubility (we will come back to this!)
Dose dependent cardiovascular depression
Reduced mortality vs Halothane
Using Sevoflurane, how does the speed of induction (unconsciousness), recovery (regaining consciousness) and also the speed at which you are able to intraoperatively modulate anaesthetic depths compare to halothane and isoflurane?
Sevoflurane is notably faster than halothane and isoflurane.
Sevoflurane licensed for all the same Isoflurane except....
It is not yet licensed in horses or rabbits.
What type of cardiovascular effect does Sevoflurane induce?
Dose-dependent cardiovascular depression similar to that of isoflurane (minimal myocardial depression with good cv stability but risk of vasodilation and reflex tachycardia).
How does the cost of Sevoflurane compare to other anesthetics?
It is more expensive.
What is the chemical formula for Nitrous Oxide?
N2O
How is nitrous oxide delivered to induce and maintain anaesthesia? Why?
You cannot induce anaesthesia with Nitrous Oxide as the MAC is too high (>100%),
so using it as an anaesthetic would mean you wouldn’t be able to provide the patient with Oxygen.
Therefore it is also popular because it can be used intraoperatively for balanced anaesthesia as an adjunct to lower inhalation agent requirements (e.g. less Sevo or Iso).
Delivered as an adjunct to oxygen, often in a mixture of 2/3 N2O and 1/3 O2.

Although not a sufficient anaesthetic when used alone, what is NO2 effective for providing? Why?
Analgesia: Activates endogenous opioids and has NMDA receptor antagonist activities (which has anaesthetic effects but in NO2's case more so its other analgesic effect- NMDA's receptors role in central sensitisation, so antagonising their action reduces the amplification of pain signals to the brain and spinal cord).
Describe the CV and respiratory effects of NO2
Can increase HR and MAP but this is transient.
Can depress respiratory system in response to elevated CO2 levels.
But mostly CVS and respiratory effects minimal.
Is Nitrous Oxide a vapour or a gas when in its gaseous form?
Nitrous Oxide mostly exits as gas in its gaseous form because its critical temperature is so close to room temperature (36.5 degrees centigrade) so it is delivered as a gas.

As it is a gas, not a vapour, how is NO2 supplied? Is this cost-effective?
Supplied in pressurised pale blue cylinders as a liquid with saturated vapour above it (the NO2 is the compressed gas because its critical temp is so close to room temp that when at room temp, this is just below its critical temp so is vapourised at room temp without any vaporiser).
- Expensive!

What are the issues associated with Nitrous Oxide?
- Risk of abuse in humans
- Long term exposure can cause bone marrow suppression and can be carcinogenic.
- Likely adds to pollution of the environment, so we must keep that in mind.

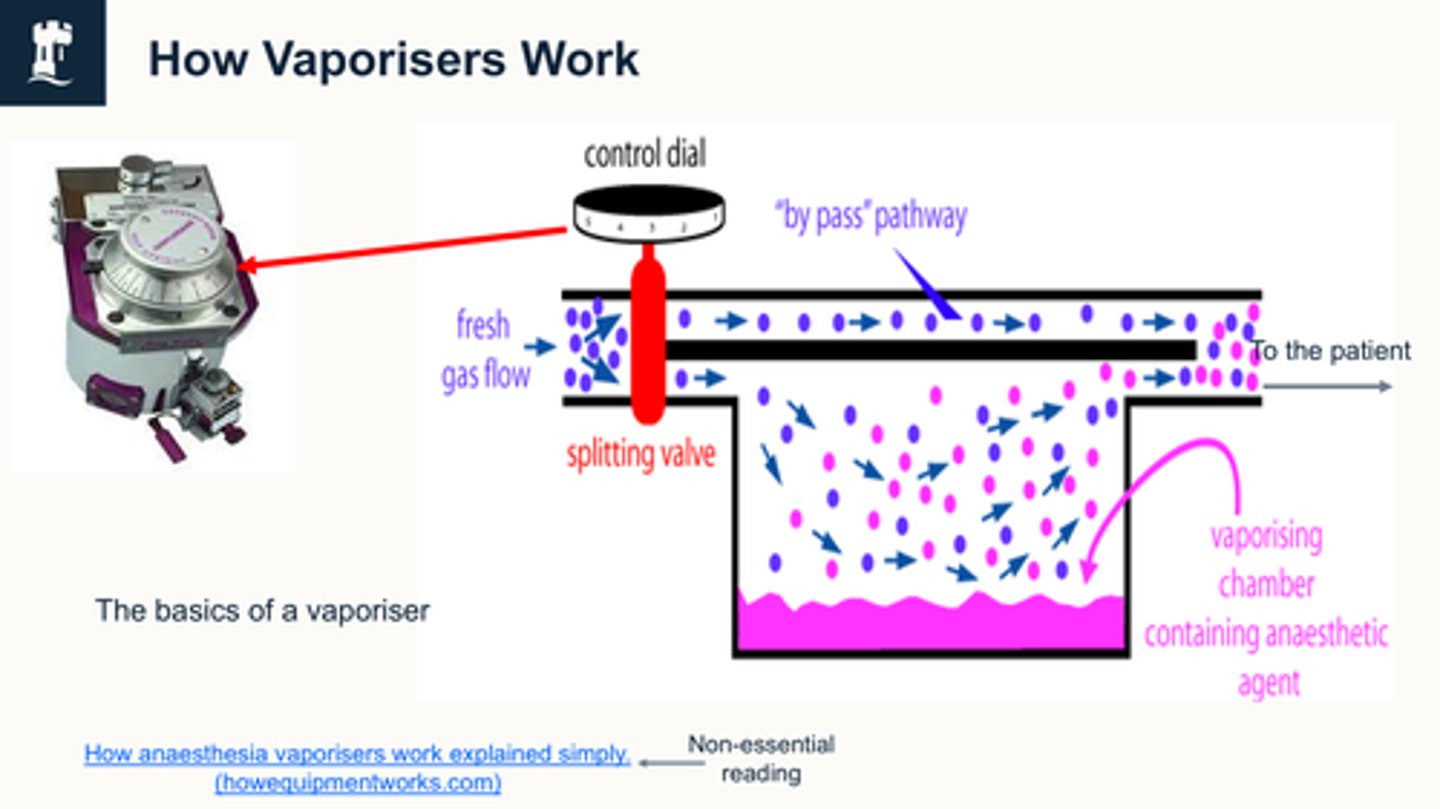
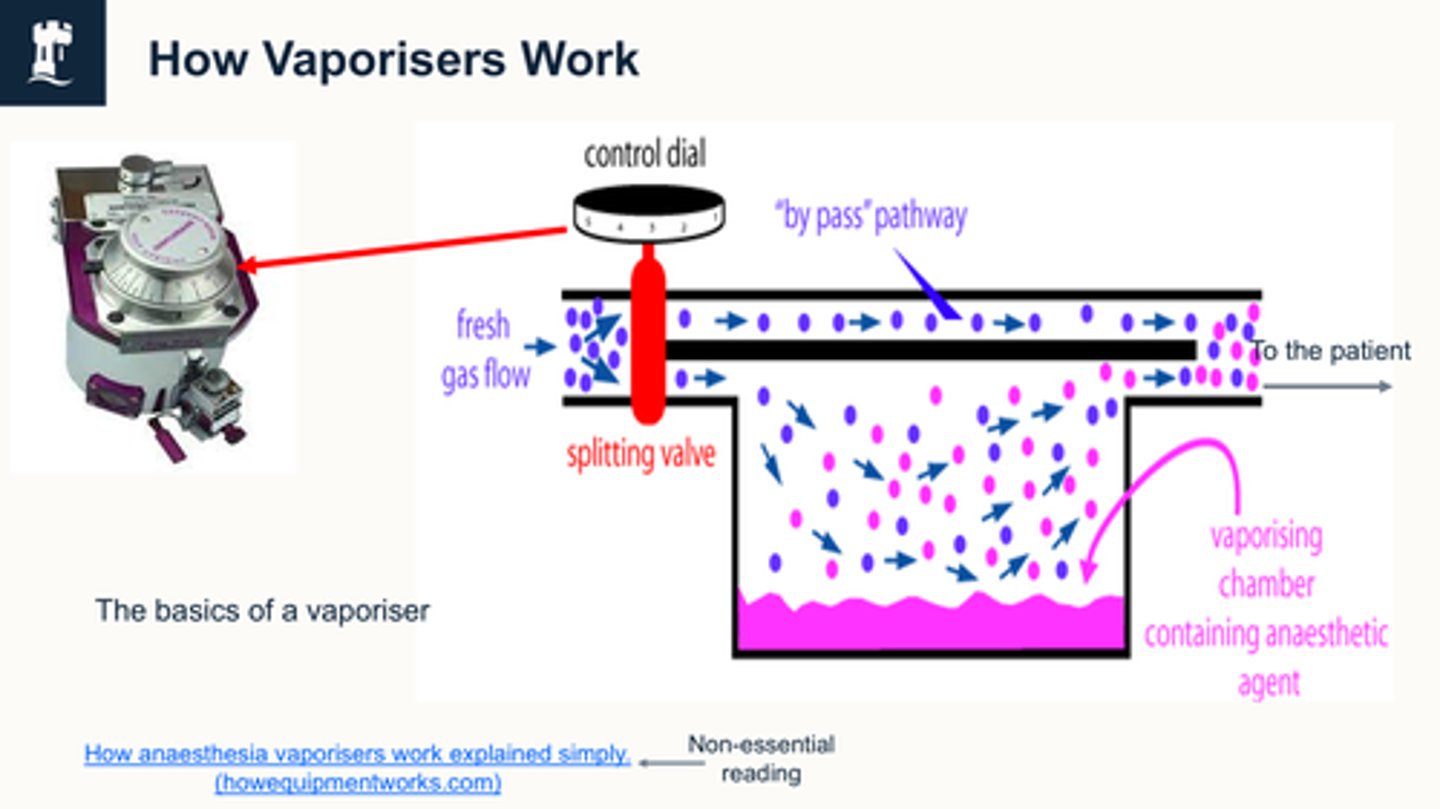
how do vaporiser work
We're changing the dial (on lhs) And if you have this dial at 0, and you have your fresh gas flow, which is your oxygen usually switched on then all of your fresh gas flow is going to bypass this splitting valve, and it's going to go straight to the patient with no inhalation agent in it
if we turn this dial on, depending, on what level we turn it on, that determines how much of the fresh gas flow, , gets split and goes this way through the vapour
the vaporizer then picks up the inhalation agent, and then they both mix at the end.
So your pure fresh gas flow and your fresh gas flow, which is your carrier gas carrying your inhalation agent, mixes here and then that goes to the patient.
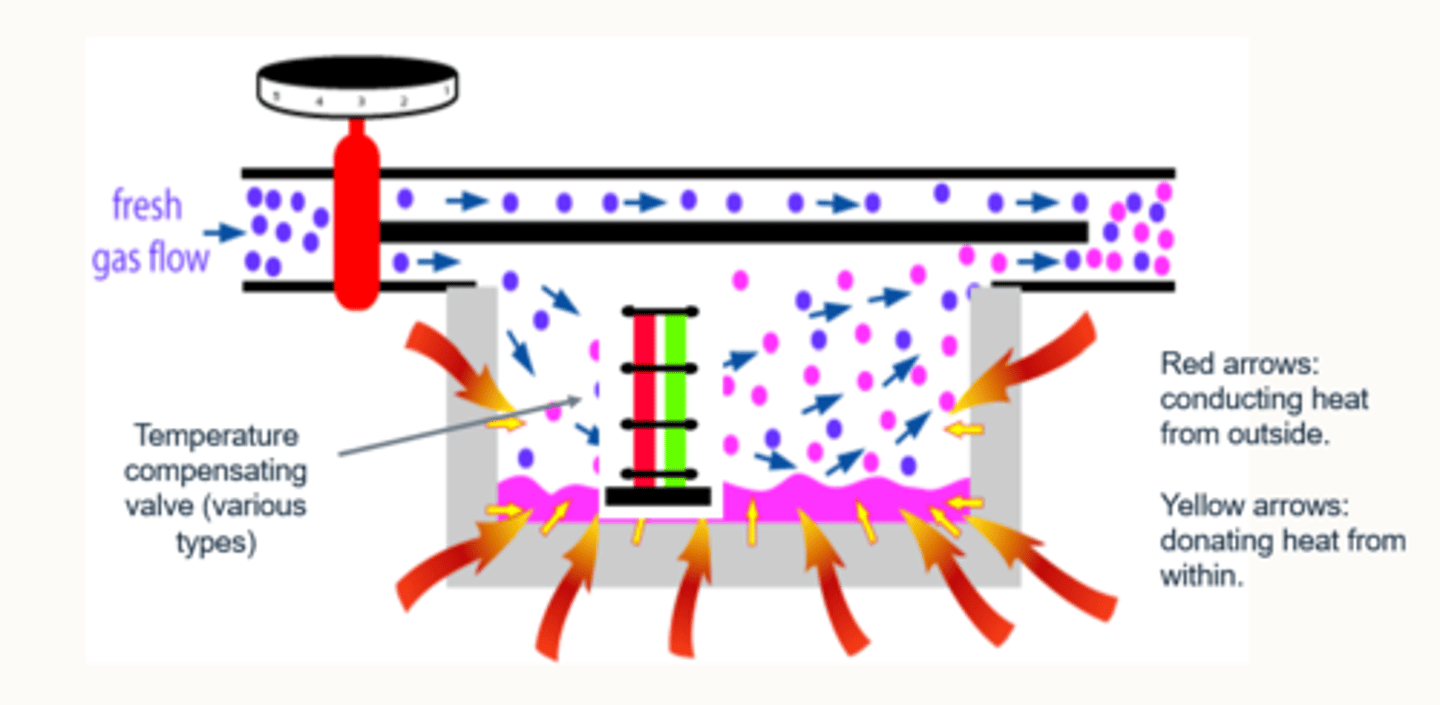
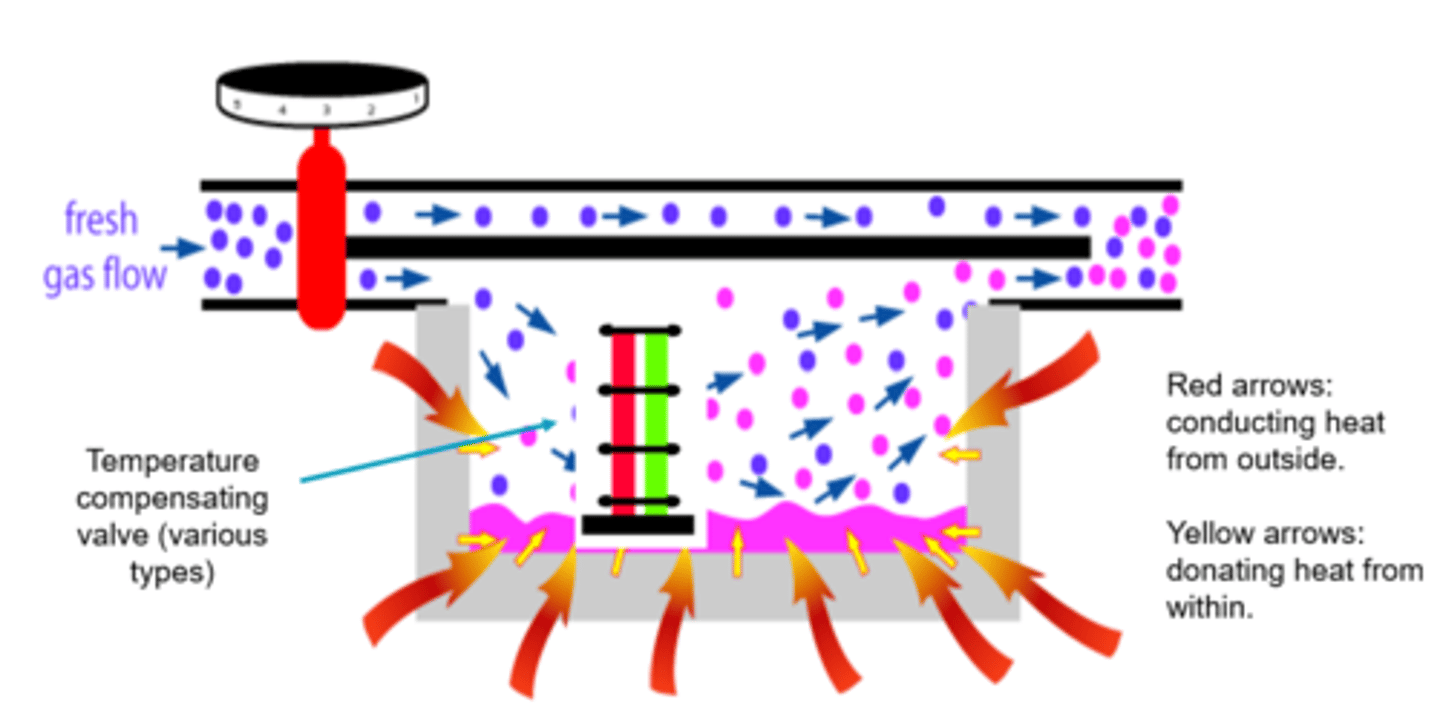
As the agent is vapourised in a vaporiser, the remaining liquid agent loses energy/temperature falls.
So if heat/energy is needed for vaporisation, how does the remaining liquid continue to be vapourised?
This is compensated by two additions to the vaporiser:
1) The vaporiser is surrounded by metal, which acts as a heat conductor, retainer and donator.
2) There are various types of temperature compensating valves which are situated within the vaporiser.
What is the main component of fresh gas flow before anaesthetic gas is added?
In a vaporizer, the liquid anaesthetic is exposed to a flow of oxygen from an oxygen cylinder that acts as a (not inert) carrier agent for the vapourised anaesthetic agent:
1) Fresh gas enters the inlet of the vaporiser and is divided into two flow pathways. The splitting valve, depending on the setting of the control dial, adjusts how much goes through each of the pathways.
2) The fresh gas that is sent along the “bypass” pathway doesn’t encounter any vapor. The fresh gas that is sent to the vaporising chamber becomes fully saturated with vapor.
3) At the exit end of the vaporiser, the bypass gas (vapourless) meets the chamber gas (fully saturated with vapor) and the two mix.
4) The resultant output depends on how much fresh gas went though each of the pathways (more fresh gas = more carrier molecules to saturate with agent), this is controlled by the vaporiser control dial. The higher the dial, the more fresh gas is sent through the splitting valve via the agent, and vice versa.
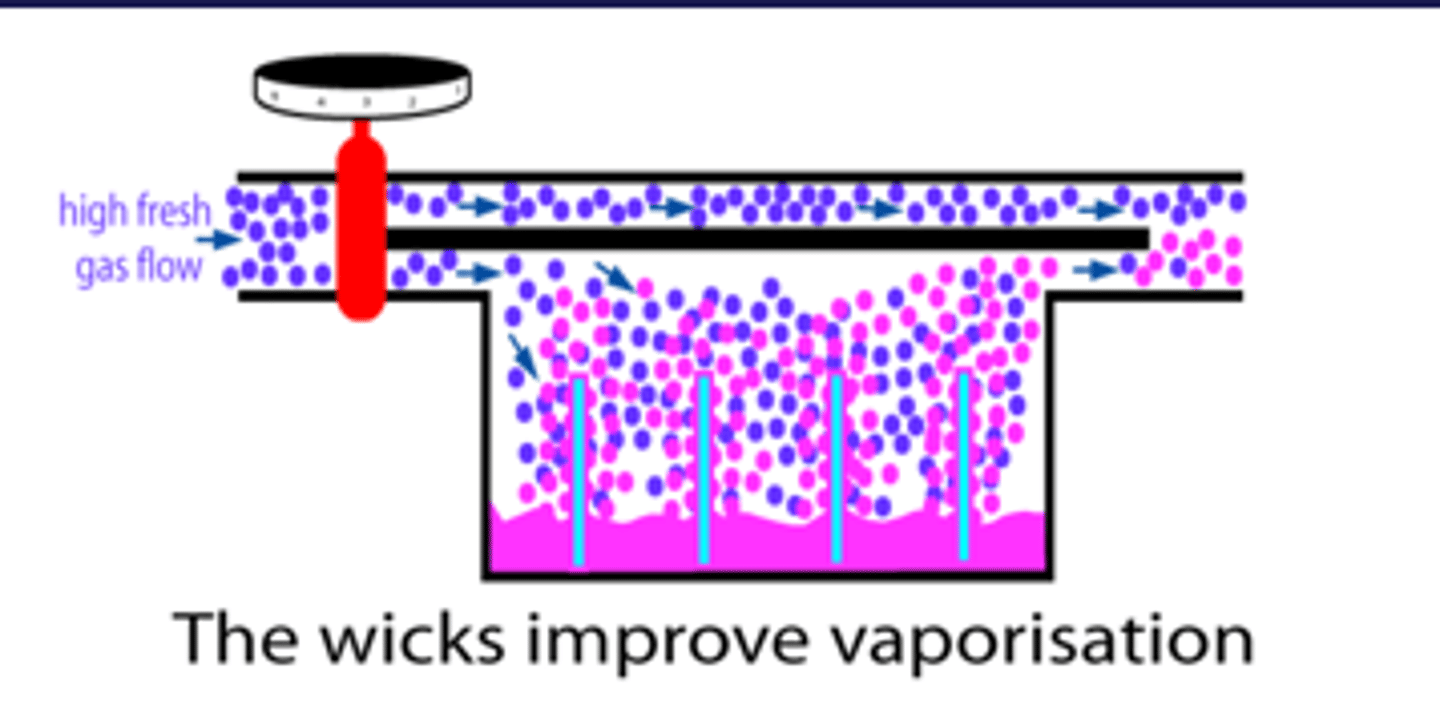
What is the function of the wick in a vaporiser?
wicks improve vapourisation (bc SA has increased)
The agent rises into the wicks, increasing the surface area of contact between the fresh carrier gas and the agent.
Without these wicks, if we set a high fresh gas flow, the vaporisation process wouldn't be able keep up with so much gas arriving in the vaporisation chamber.
The result would be that relative to the high flow of fresh gas, the amount of anaesthetic vaporised would be inadequate.
Describe temperature compensation
Activity of molecules change
Vaporises made of metal = heat conductor, retainer + donator
Temperature compensating valves
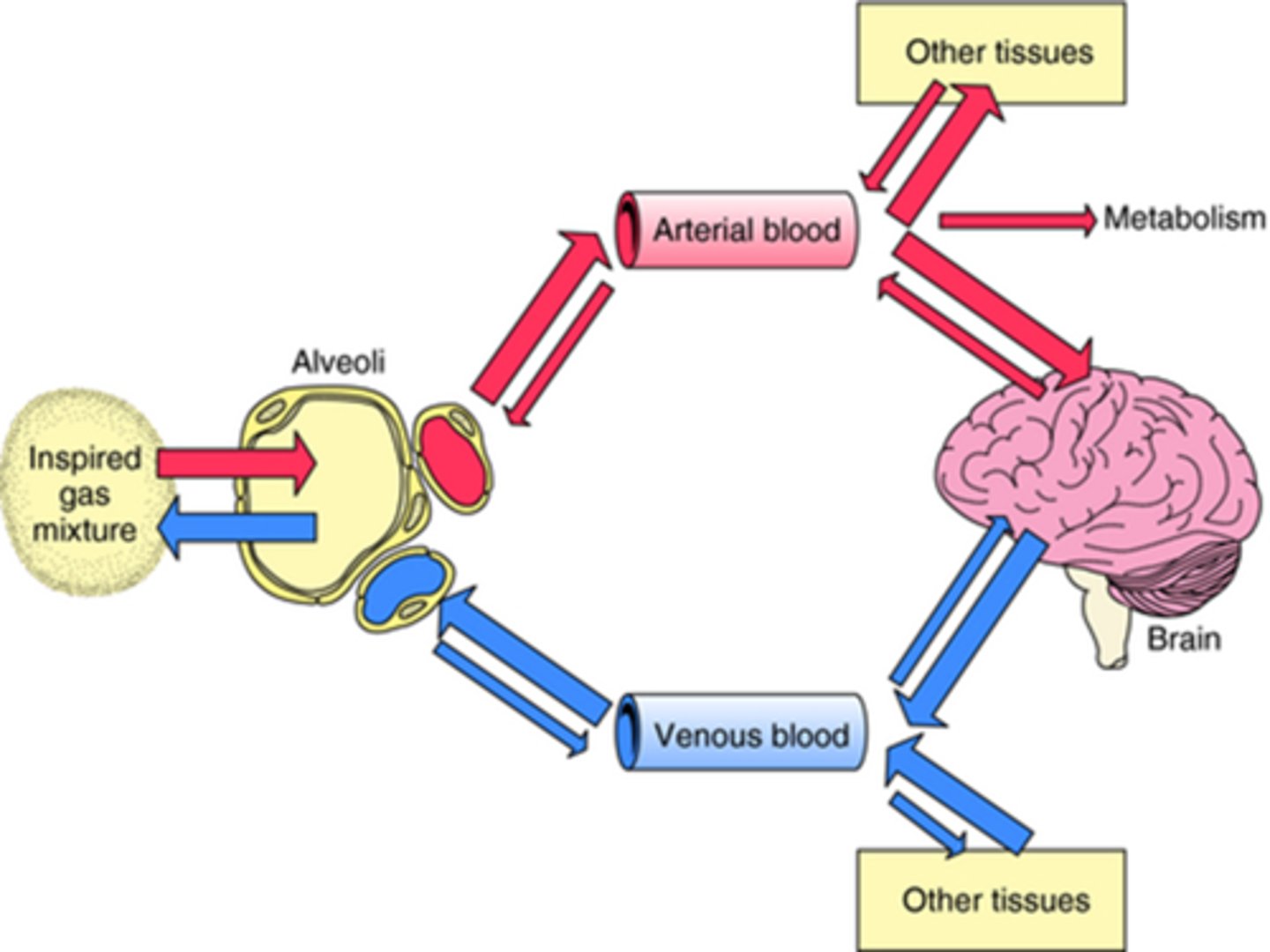
Once inhaled by the patient, how are these agents delivered?
In anaesthesia, we highjack the normal gas exchange mechanism, by inserting an endotracheal tube into the patient's trachea.
We then attach a breathing system to the ET tube and deliver 100% oxygen which acts as the carrier gas for the anaesthetic agent.
So, then, the gas exchange taking place in the alveoli now also involves the anaesthetic agent.
So when the inspired gas mixture is inhaled into the alveoli, it passes through the membrane of the alveolus into the arterial blood system, and gets transported all around the body into tissues, one of these tissue areas being the brain (crosses bbb), and that is where it causes anaesthesia.
if an agent is highly soluble in the blood how does this affect anaesthesia
then less of it is going to be in gaseous form
and cross over to the brain.
And more is going to remain in the blood
how does the amt of inhalation agent differ if patient is sick or has poor co
they often don't need much inhalation agent at all to keep them
anaesthetized because their cardiac output is
not sufficient enough to wash it out of their system.
if an agent is less soluble in the blood how does this affect anaesthesia
then more
of it is going to stay in the gaseous form
and cross over into the brain.
why can it be hard to get the right amt of anaesthesia in a young fit animal
they've got a very efficient cardiac output, which means that
they're, they're going to be very effective at washing that
isoflurane, that inhalation agent out of their system before it
had the chance to cross over to the brain.
For anaesthetic agents, when inhaled, there is also a constant exhalation of these gases too.
Why is this advantageous?
It enables us to try to maintain a constant plane of anaesthesia (get to desired level then maintained by simultaneous gain and loss of agent with inhalation and expiration).
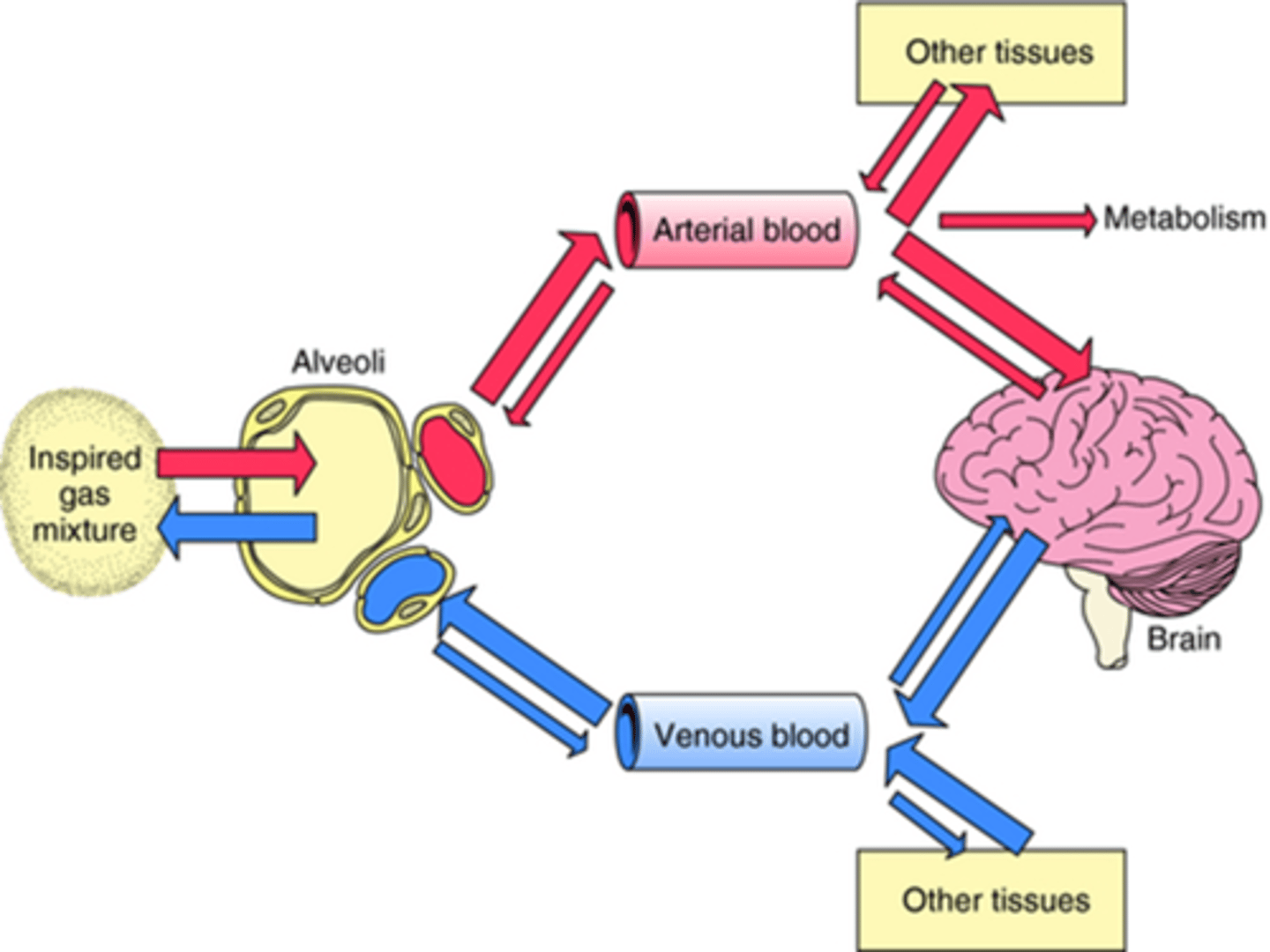
Pa
The arterial concentration of the anaesthetic.
This is crucial because the anaesthetic must reach a certain level in the blood to cross into the brain and exert its anaesthetic effects.
How quickly the inhalation agent concentration rises in plasma, depends on multiple factors, which are?
- Ventilation:
- Cardiac output
- Solubility of agent in the body
How does ventilation affect how quickly the inhalation agent concentration rises in plasma?
If the patient is breathing quickly, that will encourage exchange of the agent in the alveolus, resulting in it entering the blood more quickly. If the concentration of the agent in the alveolus is high, that will also encourage the agent to move more quickly into the blood.
Therefore, a higher ventilation rate leads to a higher concentration of anaesthetic in the alveoli, which drives the transfer of the anaesthetic into the bloodstream more quickly.
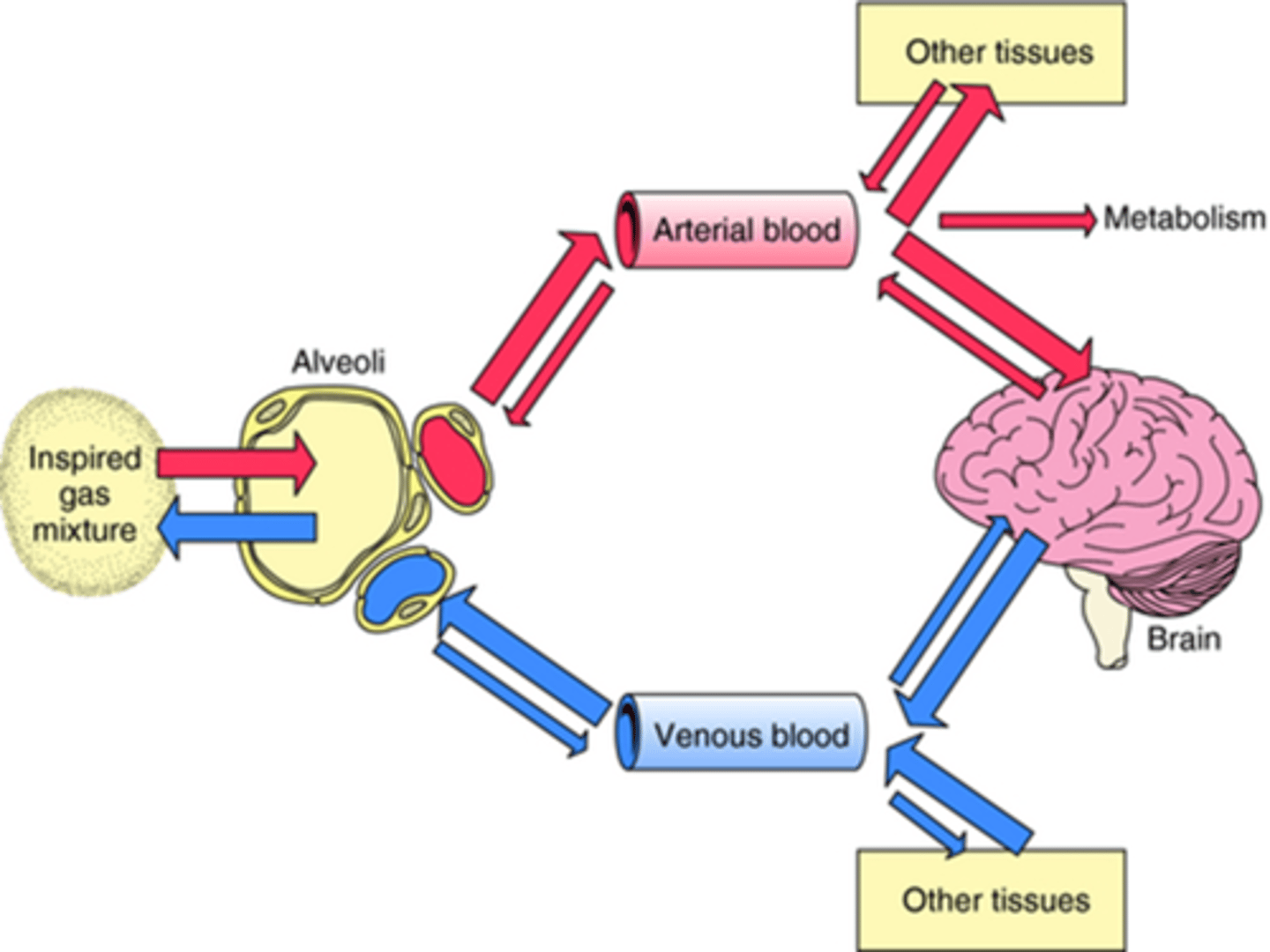
How does CO affect how quickly the inhalation agent concentration rises in plasma?
Inversely:
If CO is high, the anaesthetic is rapidly carried away from the lungs and distributed to other tissues, which can slow the rise in plasma concentration (Pa) and therefore delay the anaesthetic's action on the brain.
If CO is low, the anaesthetic stays in the lungs longer and is absorbed more quickly into the bloodstream, resulting in a faster rise in plasma concentration and a quicker onset of anaesthesia.
As a result, the induction of anaesthesia is slower because it takes longer for the agent's concentration in the brain to reach the necessary level for anaesthesia.
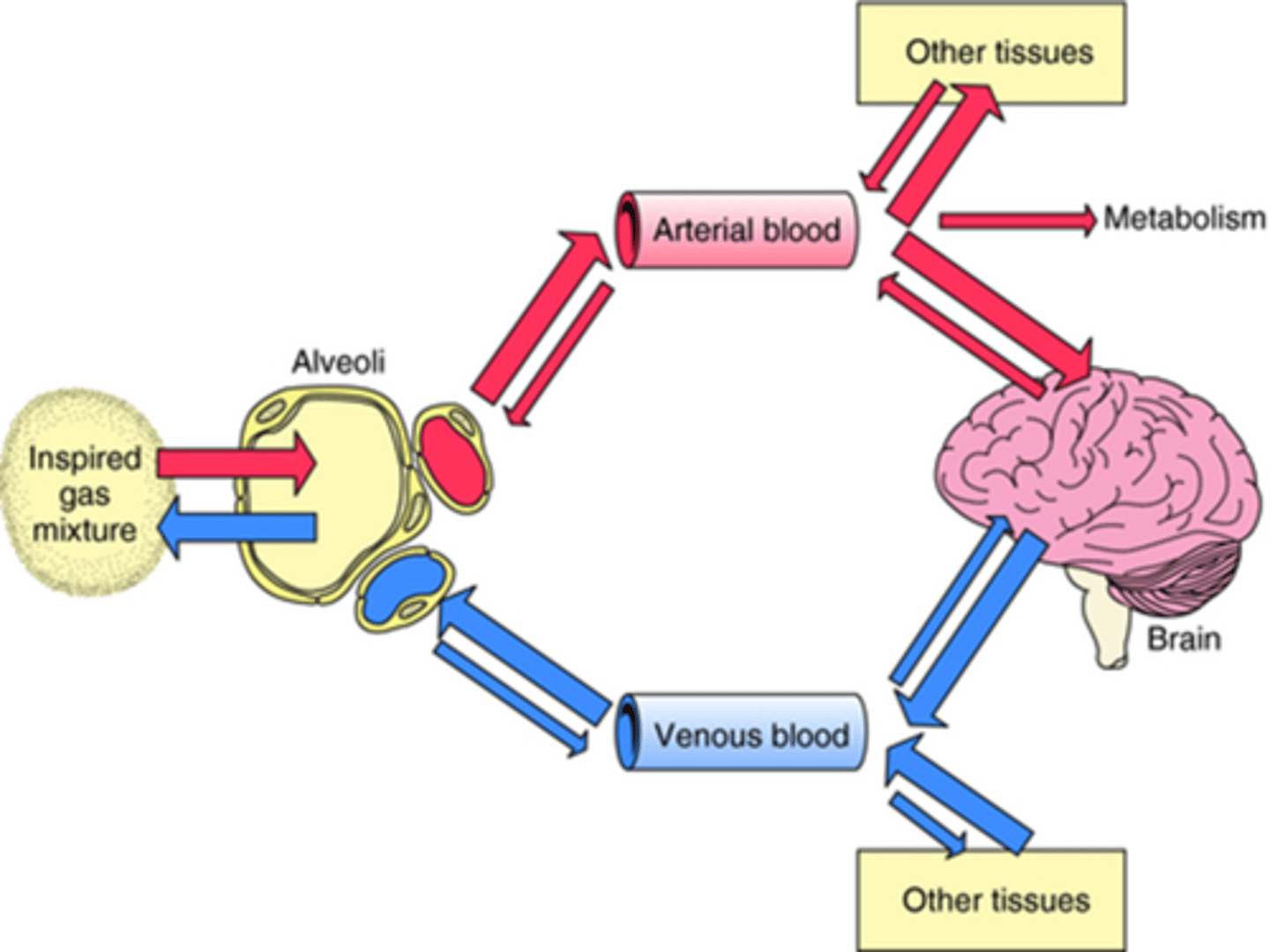
PA
Alveolar concentration of anaesthetic, an important determinant of how quickly the anaesthetic reaches the brain, as it reflects the concentration of the anaesthetic in the lungs, from where it is absorbed into the bloodstream and distributed to various tissues, including the brain.
Higher CO = faster blood flow in the alveoli = less build up of agent in the alveoli = smaller PA
but also faster blood flow = faster the agent goes from lungs to other tissues = slower rise of level in blood (Pa) needed to exert anaesthetic effect on brain.
So lower Pa can be reflected by lower PA.
How does solubility of the agent in the body affect how quickly the inhalation agent concentration rises in plasma?
Inversely: High solubility slows induction by delaying the rise in PA because the blood "holds onto" the agent, slowing its transfer to the brain and reducing the speed of induction.
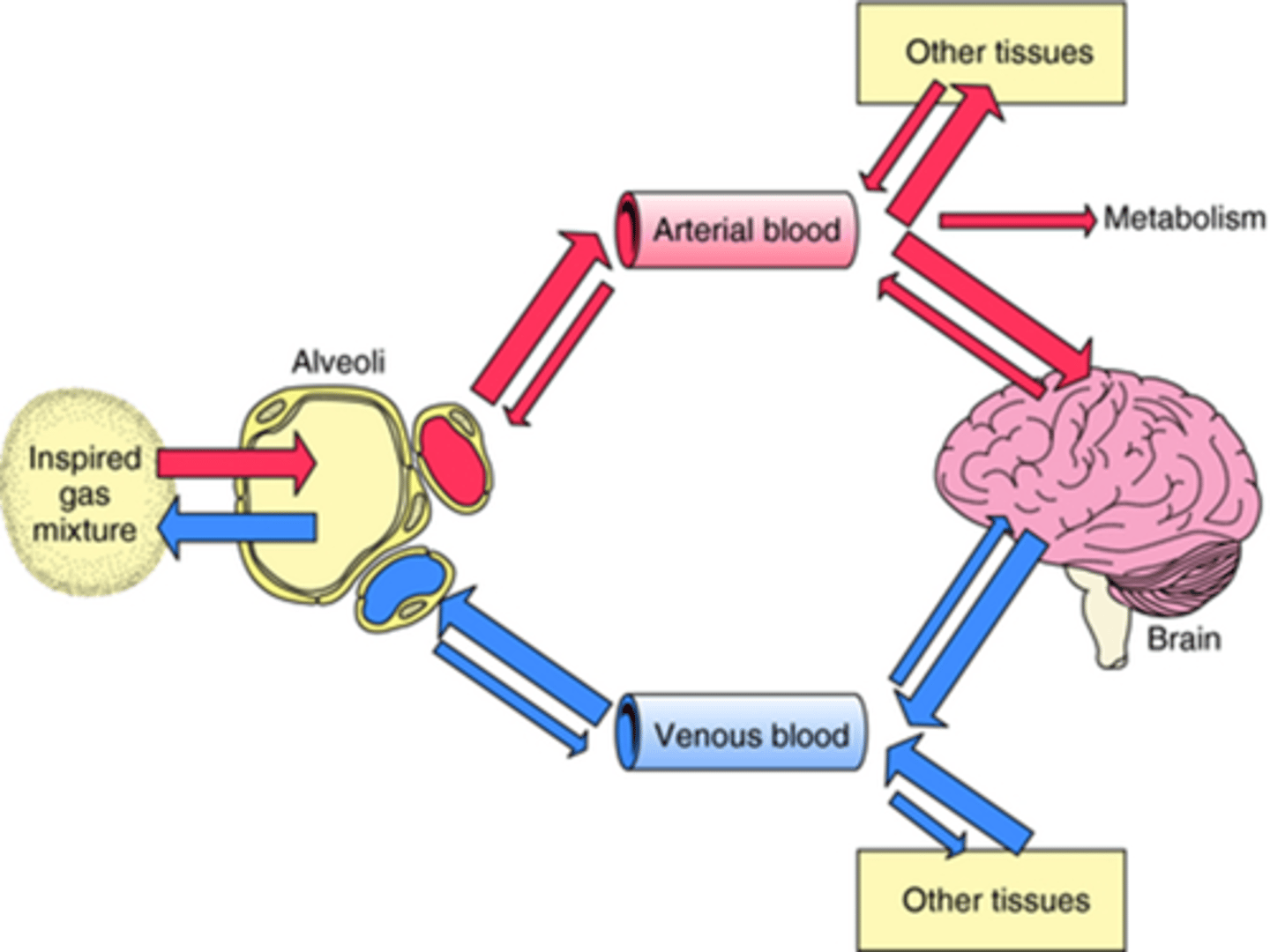
How does being young or sick effect concentration of inhalation?
Young fit animal:
- high CO => slower rise in Pa => slower to anaesthetic effect (need more agent)
Very sick patient
- low CO => faster rise in Pa => faster to anaesthetic effect (need less agent)
what is MAC
The concentration of a vapour in the alveoli of the lungs that is needed to prevent movement in 50% of subjects in response to surgical stimulus.
what is MAC used to compare
MAC is used to compare the strengths/potency of anaesthetic vapours, but can also be used to contribute towards decision making during anaesthesia.
represents a guide not a target
what changes an animals MAC
anything that speeds up the rise of Pa (like decreased CO) and thus speeding up the time it takes anaesthetic onset means that animal's MAC can be smaller and vice versa (fit animals).
OBESITY IS THE EXCEPTION TO THIS RULE AS IT HAS DELAYED ONSET BUT NOT INCREASED MAC ALONGSIDE THIS
what are MAC measures determine in, what does this mean
healthy, un-pre-medicated patients,
so in theory, considering all the premedication drugs we give, our patients shouldn’t require much more than the MAC value for that species.
MAC is species specific and can also be individual-specific; there are ways of working out MAC for each individual patient→ So, we use these values as a rough guide
What are the MAC values in cats and dogs?
Isoflurane: 1.28 dog, 1.63 cat, 1.3% horse, 0.9% foal
Sevoflurane: 2.2 dog, 2.58% cat, 2.3% horse
Desflurane: 10.3 dog, 9.8 cat
Nitrous oxide: 188-297 dog, 255 cat
What factors do not influence MAC?
Anaesthetic duration
Atropine, glycopyrrolate
Metabolic acid-base change
Hyper + hypokalaemia
Gender
High MAP
Hypo/hyperthyroidism
What factors influence an increase in MAC?
- Strong cardiac output (usually on induction),
- young/fit animal
- Drugs causing CNS stimulation e.g., ephedrine, various others
- Hyperthermia
so give more to keep anaesthetised
what is MAC like for rabbits
is much higher so need more anaesthetic
What factors influence a decrease in MAC?
Old age
Disease
Pregnancy
Hypothermia
Analgesics, opioids, Alpha 2, ketamine, N20, LA, and other drugs e.g. BZD + ACP (synergistic action alongside inhalant means less of inhalant needed for anaesthetic effect = reduced MAC)
local anaesthetics
Low MAP (hypotension)
so need less to keep anaesthetised
Describe the impact of being overweight vs lean on the onset of induction
obesity generally leads to an increased CO, so a slower rise in Pa.
Generally anything that slows the rise of Pa (like increased CO) and thus the slows speed of time it takes to anaesthetic onset means that animal's MAC needs to be larger (and vice versa).
However, MAC for obesity is actually decreased, this is because this CO effect is counteracted by the sequestration of fat of the inhalation agent, most of which are lipophilic.
This means less is available to the brain, so may delay the onset of anaesthesia like CO, but obesity will not increase the MAC alongside this delayed onset because the when more anaesthetic is stored in fat, the initial concentration in the brain may be lower, but the overall effect can still be achieved with less anaesthetic over time, because the fat acts as a reservoir.
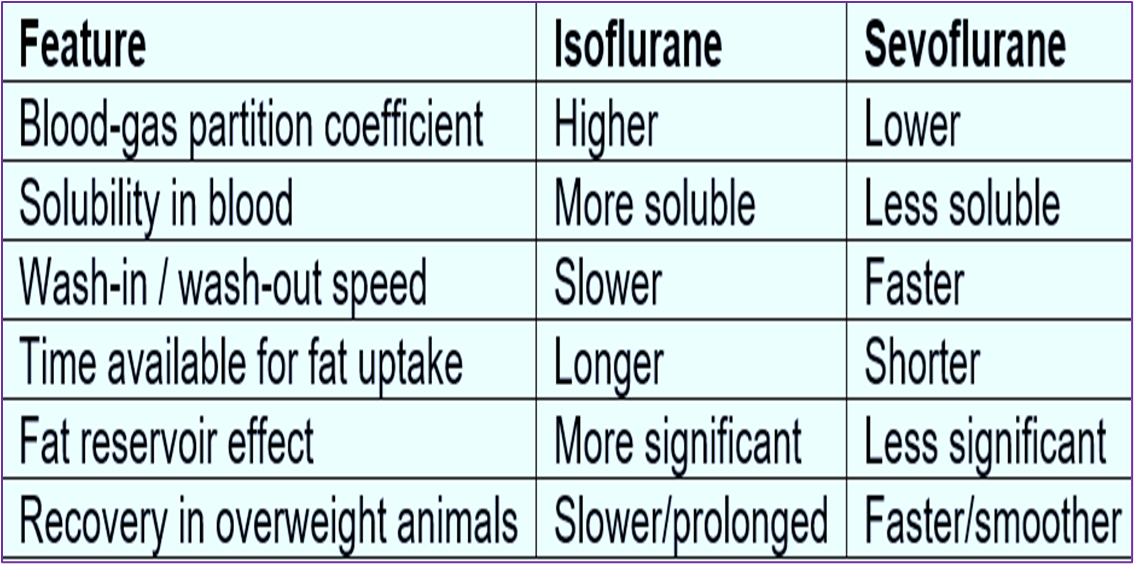
Describe the impact of being overweight vs lean on recovery
Recovery is the reverse of induction, redistribution will have occurred into the fat, which then acts as a reservoir for anaesthetic so (depending on fat solubility) an overweight animal will recover slower than a lean one.
bc will leave from fat back into the blood stream
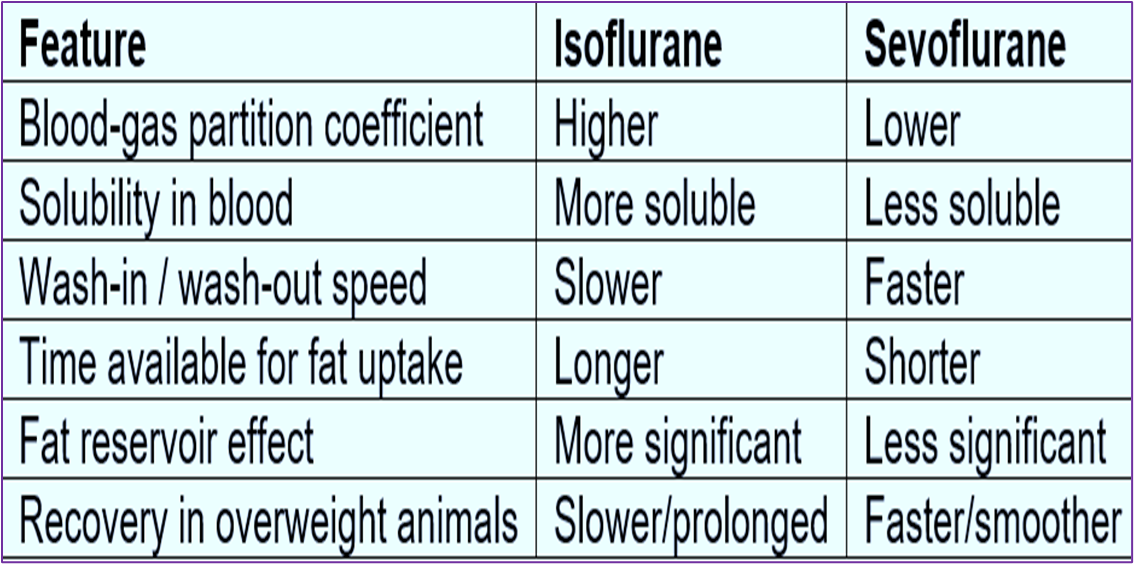
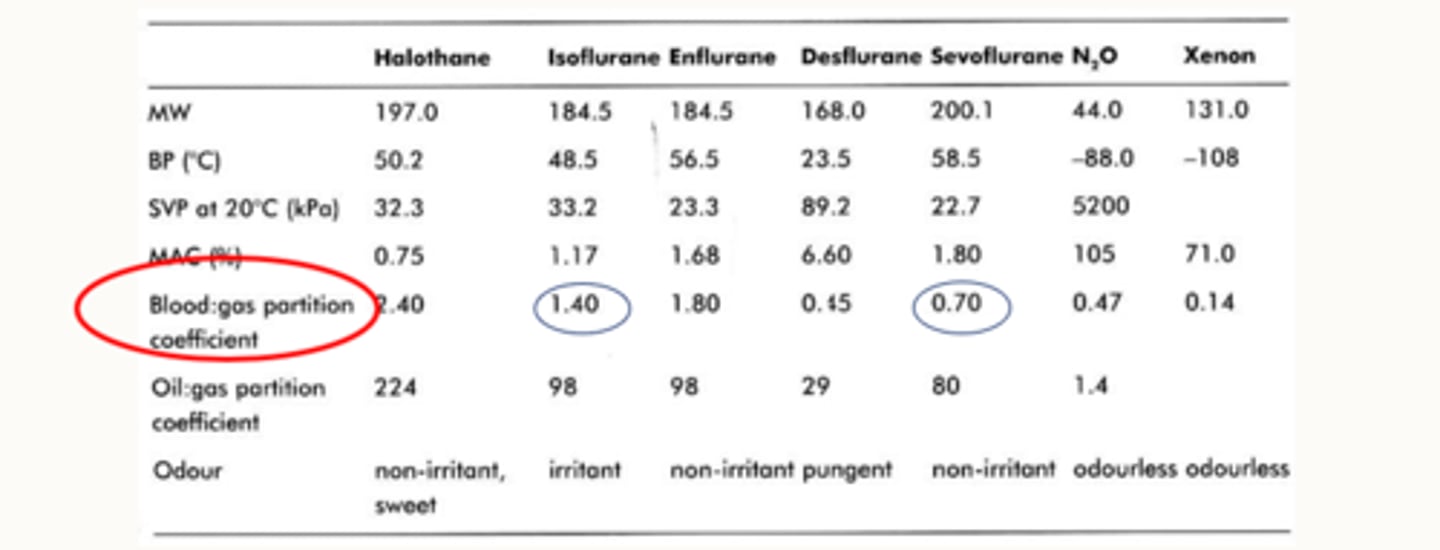
Describe the blood : gas partition coefficient
Blood : gas partition coefficient = Solubility
This is the ratio (shown in a non-ratioed value) of the amount of anaesthetic in blood and gas when the two phases are of equal pressure and volume (how much of the agent is dissolved in the blood and how much is in a gaseous form just in contact with the blood).
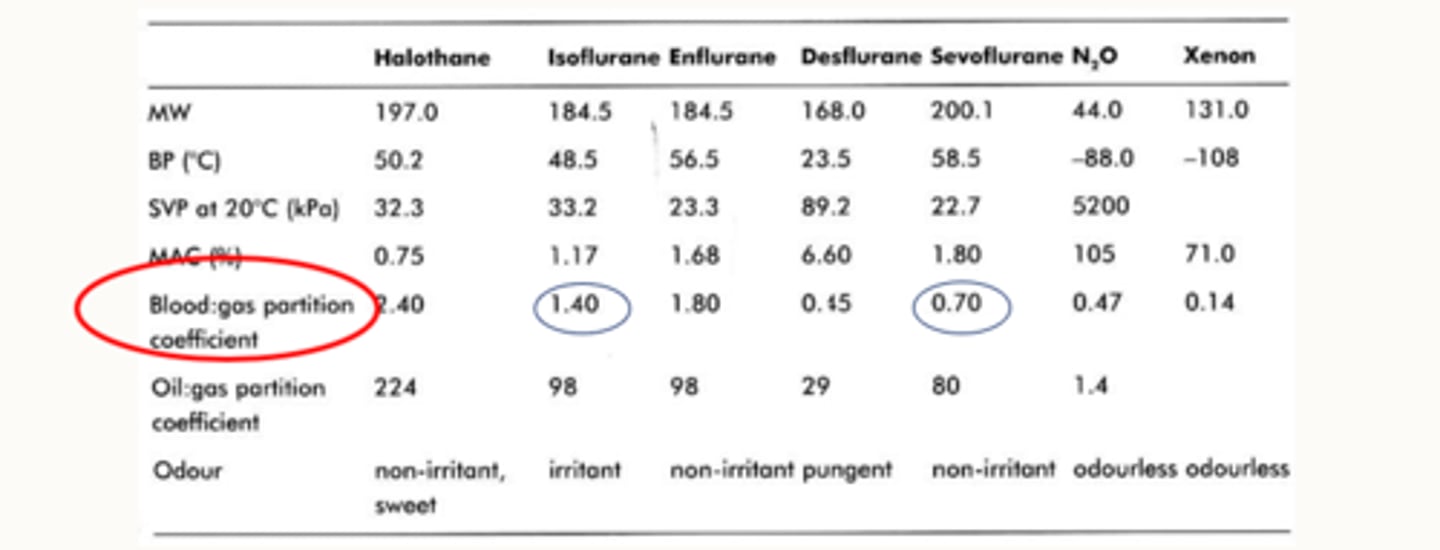
how does the blood gas partition coefficient for iso and sevo differ
the blood gas partition coefficient for iso is a is a lot higher than sevo
so a lot of iso stays in the blood.
That means less of it crosses over
So that means when you make changes by increasing or decreasing your dial, it might take a little bit longer to see those changes happen compared to sevo which has a much lower blood gas solubility
Agent dissolved in blood is bound to proteins (more soluble) which inhibits the anaesthetic from crossing the blood-brain barrier and having its effects (we still don't have a good idea how these work either).
So, do anaesthetics with low blood-gas coefficients have a quicker or a slower onset (induction)?
Anaesthetics with low blood-gas coefficients have a quicker onset (induction) because more of the anaesthetic is in gas form rather than dissolved (less soluble), this will also mean a quicker recovery, because there will be less of the agent bound to proteins in the bloodstream.
So Sevo, theoretically has a faster induction than Iso because its got a lower blood : gas partition coefficient (less soluble in the blood).
How do inhalation agents work?
Mostly unknown! mechanism not fully understood
Fundamentally, they work within the CNS, by augmenting signals to chloride channels (GABA receptors) and potassium channels (2PK) while depressing neurotransmission pathways. The jury is still out on this!
All inhalation agents cause dose-dependent cardiovascular depression (reduced MAP, HR, SV, CO).
How do they do this?
decrease bp and CO via
By direct myocardial depression (negative inotropy)
By causing peripheral vasodilation
By decreasing vascular reactivity and impairing tissue autoregulation (loss of baroreceptor reflex, tissue perfusion becomes more dependent upon the “driving” (systemic arterial BP)
Via CNS depression and reducing autonomic tone
ALL DOSE-DEPENDENT!
All inhalation agents cause dose-dependent respiratory depression.
How do they do this?
• By a depressed ventilatory response to CO2
• By a depressed hypoxic pulmonary vasoconstriction
• By almost abolishing the ventilatory response to hypoxia
• By causing bronchodilation (which increases dead space)
ALL DOSE-DEPENDENT!
how to mitigate negative effects of inhalation agents
Practicing balanced anaesthesia (multiple drugs at small doses rather than large amount of one agent (particularly volatile agent/PIVA).
MAC sparing drugs
partial intravenous anaesthesia
Negative effects of inhalation agents on humans
- Little or no evidence to prove there are significant negative effects with most agents as studies contradict each other so the jury is still out. → but presume it is
- Some studies suggest increased miscarriages in people working in surgical theatres; other studies suggest differently.
- However, there is hard evidence that long-term exposure to nitrous oxide can cause bone marrow suppression and teratogenesis (congenital malformations in the foetus).
- immunocompromised
- pregnant
- uncomfortable
What are the properties of an ideal inhalation agent?
• Stable
• Preservative free
• Non-inflammable
• Cheap
• Ozone friendly
• Non metabolised
• Non-toxic
• CVS effects free
• Analgesic
but not real
What gas may be used as an ideal inhalation gas for anaesthesia in the future?
Xenon exists as a gas at room temperature, or approximately 23°C. Xenon's boiling point is about 165.1 K (around -108.1°C or -162.6°F). At temperatures below its boiling point, xenon becomes a liquid. Once temperatures reach its melting point, which is about 161.4 K (approximately -111.8°C or -169.2°F), Xe exists as a light-blue solid.
Describe the use of scavenging + COSHH
Passive and active scavenging
Annual audits to measure exposure
Careful filling of vaporisers (special keys)
Flushing breathing systems before disconnection
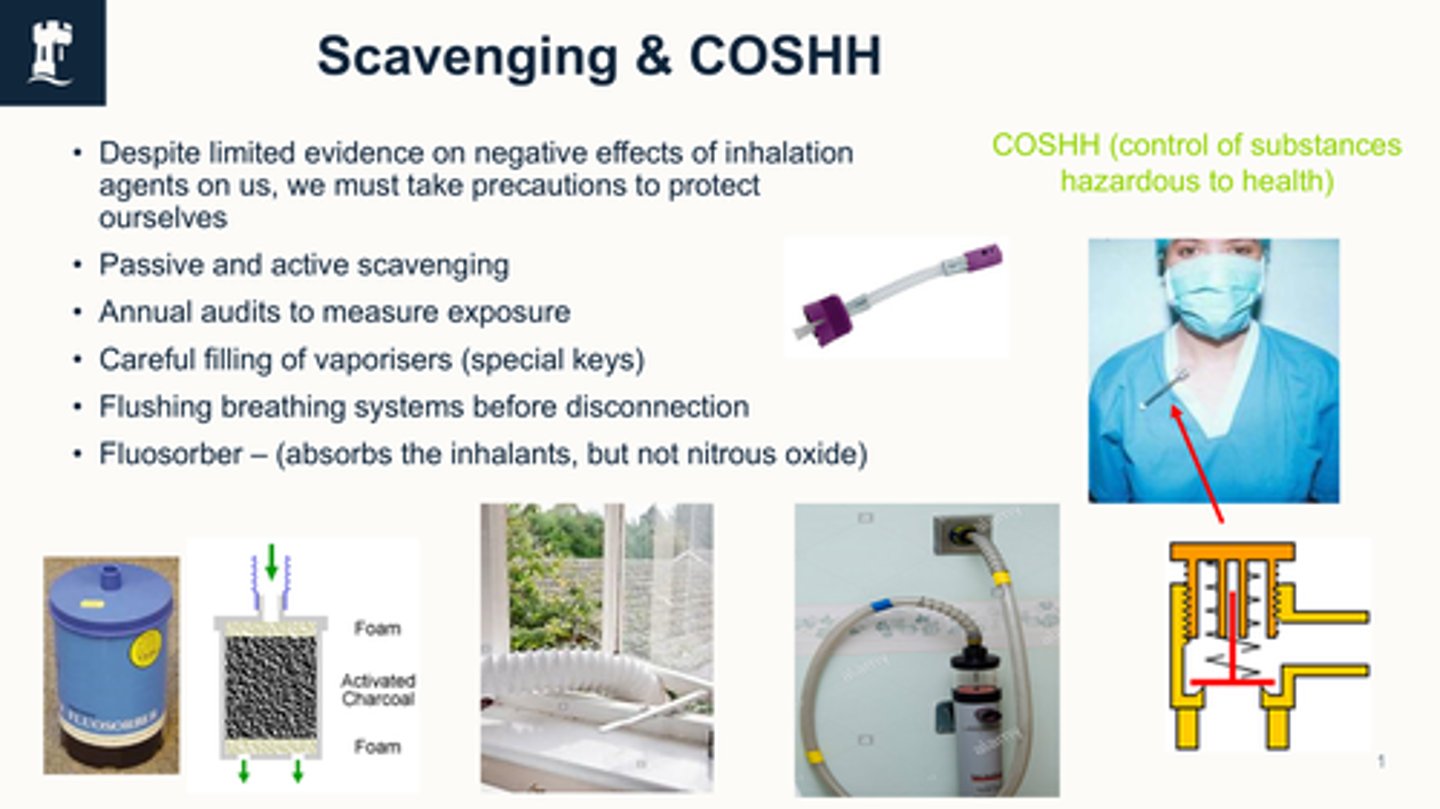
Fluosorber is a passive scavenger - (absorbs the inhalants, but not nitrous oxide)
VCT (volatile capture technology) captures and absorbs anaesthetic agents that would otherwise be released into the environment.
Early stages of use currently.
what is COSHH
legal framework governing exposure to hazardous substances
what are passive scavengers
relies on the patient’s exhaled gases being directed away from the breathing system without suction
Get to a certain weight and it goes into clinical waste to be incinerated
use activated charcoal → only adsorb volatile anaesthetic agents only and do not remove nitrous oxide.
must be on floor
what is active scavenging
uses a dedicated vacuum or suction system to actively remove waste anaesthetic gases from the breathing system and extract them from the clinical environment
safer
why do passive scavengers need to be kept on the floor
they work by gravity - waste gases weigh more.
what is VCT (volatile capture technology)
Captures and absorbs anaesthetic agents that would otherwise be released into the environment.
Early stages of use currently.
Evidence of benefits is clear, but further research is required to improve understanding of utility.
Important facts about equine anaesthesia using inhalants
higher anaesthetic risk
Horses are prone to hypotension when inhalants are used
Inhalational agents cause marked cardiopulmonary depression
Prolonged hypotension is likely to cause post op myopathy
Post op myopathy may necessitate euthanasia or cause a fracture or injury in recovery
TIVA or PIVA may reduce hypotension, but both have limitations
lower mortality with TIVA than inhalational anaesthesia
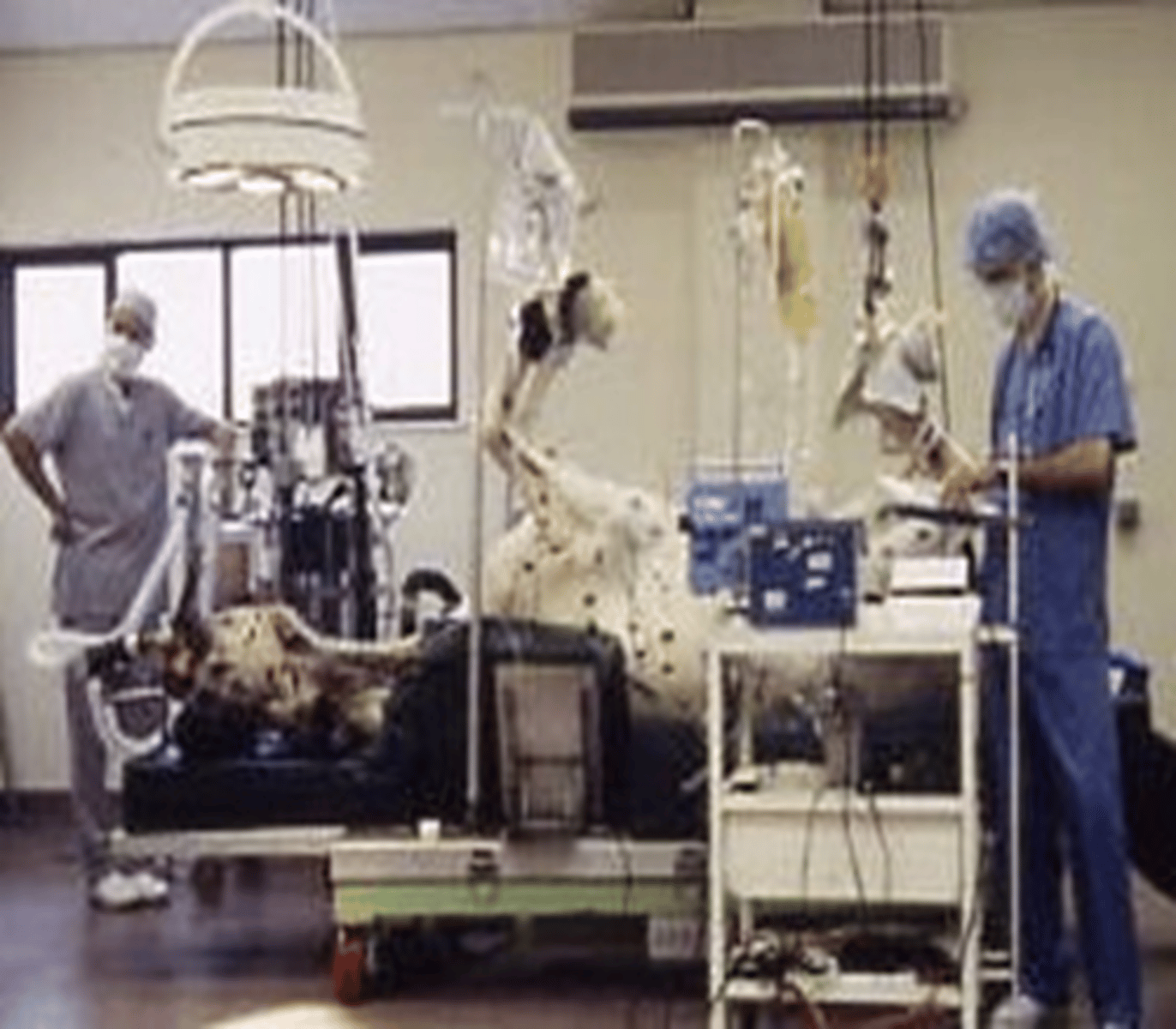
Describe TIVA
Total intravenous anaesthesia
2 ways:
- top-up boluses (propofol, alfaxalone, ketamine)
- continuous rate infusions (propofol, alfaxalone, triple drip in equine)
what is the goal of tiva
Goal of TIVA: No inhalation agent! Produces a much-diminished anaesthesia stress response compared with inhalation agents and therefore, considered a physiologically superior method of anaesthesia.
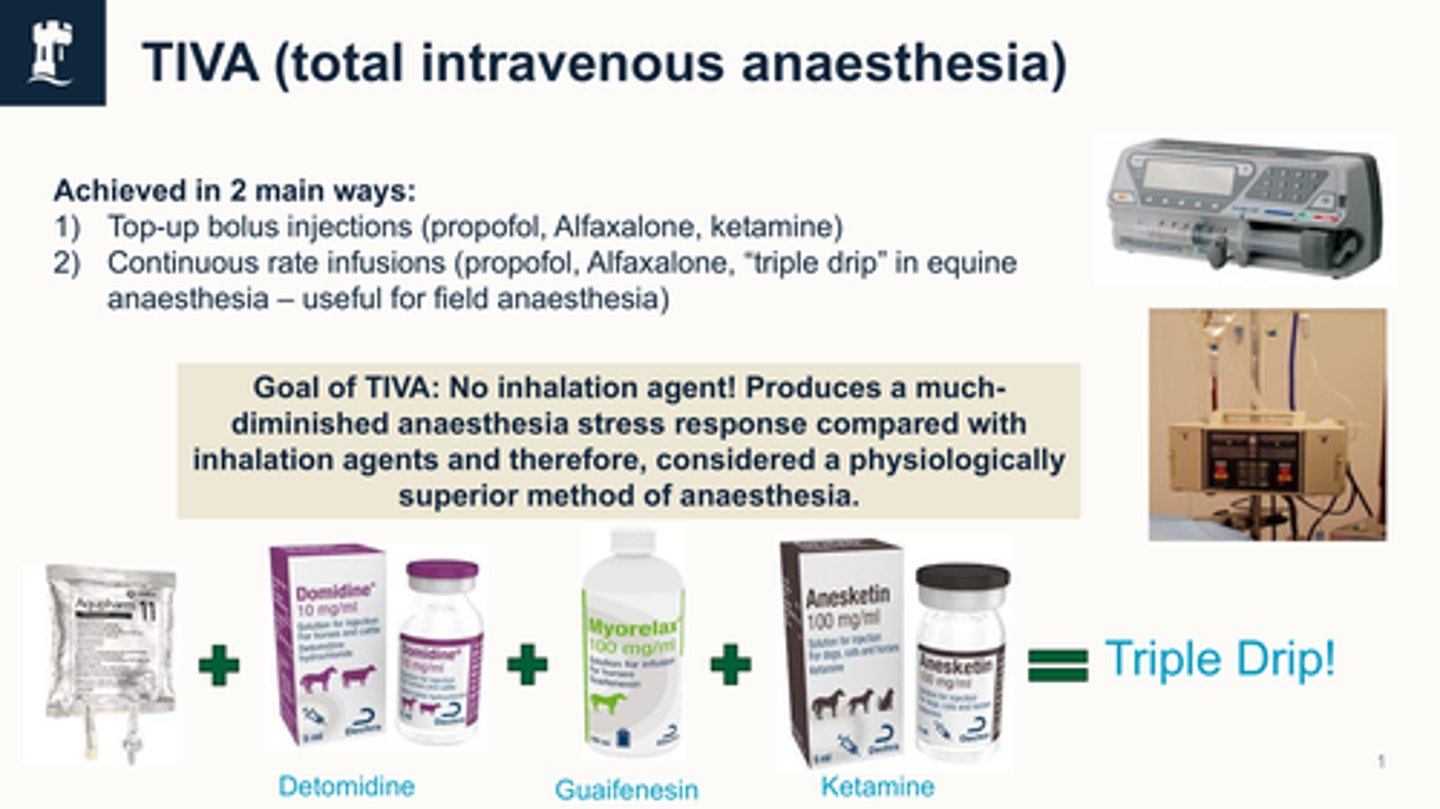
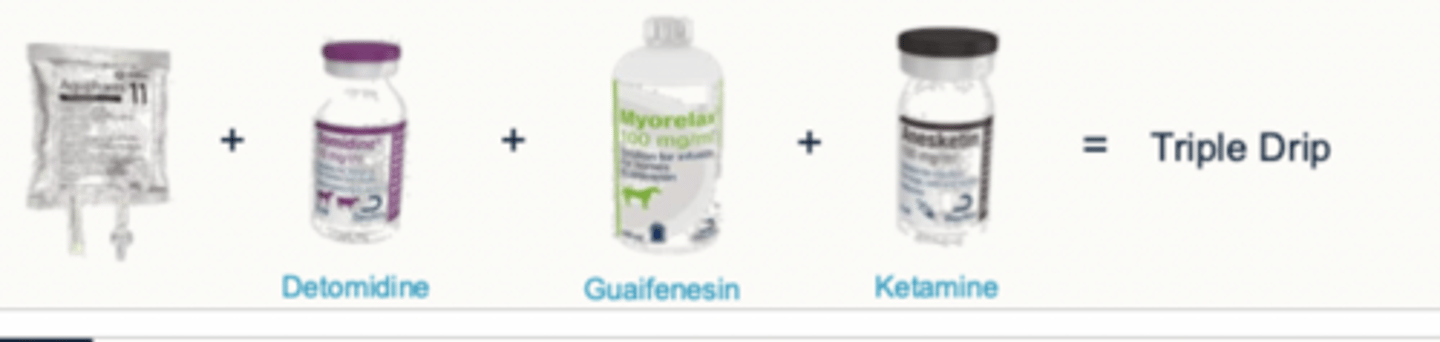
what is the triple drip (TIVA)
Triple drip = Aquapharm + detomidine + guaifenesin + ketamine
common in equine
what is PIVA
Partial intravenous anaesthesia
PIVA recipes:
- inhalant + lidocaine (CRI)
- inhalant + ketamine (CRI/top-up)
Lidocaine + ketamine have been shown to improve CV stability during isoflurane anaesthesia.
- inhalant + alpha 2 agonist (CRI)
- inhalant + opioid
what are the goals of PIVA
Reduce MAC
Reduce cardiopulmonary depression
Provide additional analgesia
Contributes to balanced anaesthesia
Less pollution
Better outcome? The foreseeable future?
What are some downsides to PIVA?
- Cardiopulmonary depression will still occur
- Most IV drugs accumulate over time
- Additional equipment required

How can we minimise the negative effects of inhalation agents?
•Calculate fresh-gas-flows correctly
•Thoroughly check all anaesthetic equipment before use
•Practice "balanced anaesthesia" (i.e. multiple drugs at small doses rather than large amounts of one, particularly inhalation agents)
•Know your drugs (i.e. MAC sparing, induction agent sparing etc.)
•Anaesthetic plans (prompts you to calculate and prepare "back-up" drugs in case of nociception), also helps you to be organised, hopefully avoiding longer than necessary anaesthetics.
•Diligent monitoring of patient from beginning to end!
•TIVA/PIVA
TIVA
total intravenous anaesthesia
top-up bolus injections (propofol, alfaxalone, ketamine)
continuous rate infusions (propofol, alfaxalone, triple drip anaesthesia in equine
what is the goal of TIVA
no inhalation agent
produces a much diminished anaesthesia stress response
physiologically superior method
PIVA
partial intravenous anaesthesia
lidocain CRI
ketamine CRI or top ups
Alpha-2-agonists
opioids
goals of PIVA
reduced MAC
reduced cardiopulmonary depression
provides additional anaesthesia
balanced anaesthesia
less polution
better outcomes
Summary