Honors Physical Science Midterm Review
1/54
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
55 Terms
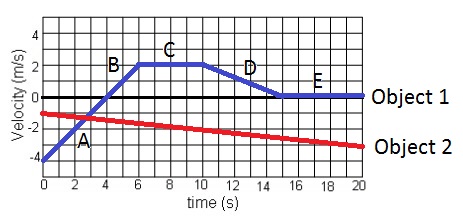
At which time frame is object 1 motionless for the entire segment of time?
A) 4-6 seconds
B) 6-10 seconds
C)10-15 seconds
D) 15-20 seconds
15-20 seconds
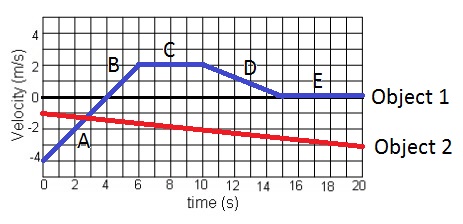
What is true about both objects when their graphs intersect just before 3 seconds?
A) they both have the same position
B) they both have the same velocity
C) they both have the same acceleration
D) all of the above
E) none of the above
B) they both have the same velocity
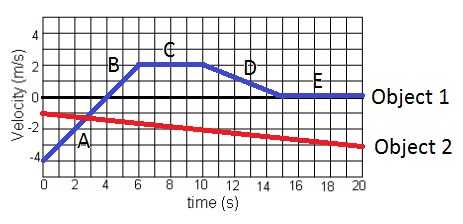
What time frame on the graph does object 1 have positive acceleration?
A) 0-6 seconds
B) 6-10 seconds
C)10-15 seconds
D) 15-20 seconds
E) none of the above
A) 0-6
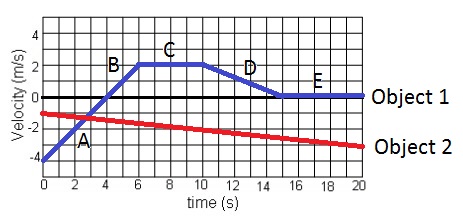
What time frame is the velocity of object 1 constant?
A) 0-6 seconds
B) 6-10 seconds
C)10-15 seconds
D) 15-20 seconds
E) none of the above
B) 6-10 seconds
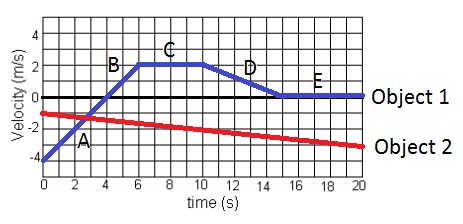
What is the acceleration of object 2 for the time interval 0-10 seconds?
A) 0.1 m/s
B) 0.2 m/s
C) 0.1 m/s/s
D) -0.2 m/s/s
E) -0.1 m/s
F) -0.2 m/s
G) -0.1 m/s/s
G) -0.1 m/s/s
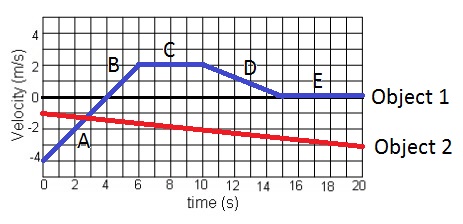
What is true of both objects from the time interval 10-14 seconds?
A) Both objects have a positive velocity.
B) Both objects have a positive acceleration.
C) Both objects have a negative velocity.
D) Both objects have a negative acceleration.
D) Both objects have negative acceleration.
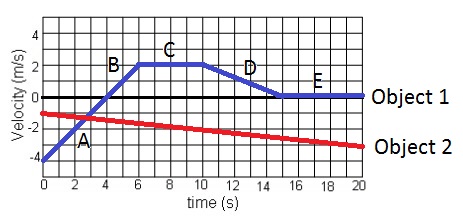
What is the velocity of object 1 at time = 10 seconds?
A) 0 m/s
B) 2 m/s
C) -2 m/s
D) 2 m/s/s
E) -2 m/s/s
F) None of the above
B) 2 m/s
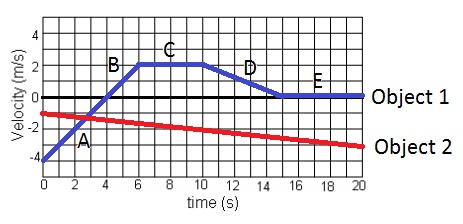
What is the acceleration of object 1 from 0-6 seconds?
A) 0 m/s
B) 2 m/s
C) 6 m/s
D) 1 m/s
E) -1 m/s
F) -2 m/s/s
G) -6 m/s/s
H) 1 m/s/s
I) -1 m/s/s
J) None of the above
I) -1m/s/s
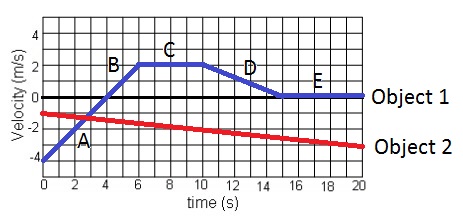
What time frame is the acceleration of object 1 equal to 0 m/s2? Select all that apply.
A) 0-6 seconds
B) 6-10 seconds
C) 10-15 seconds
D) 15-20 seconds
B) 6-10 seconds & D) 15-20 seconds.
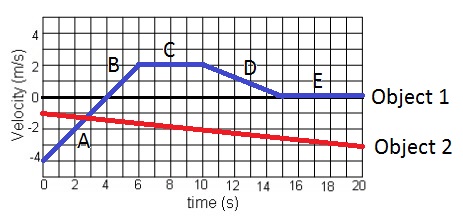
What time frame does object 1 have a negative velocity? Select all that apply.
A) 0-4 seconds
B) 6-10 seconds
C) 10-15 seconds
D) 15-20 seconds
A) 0-4 seconds
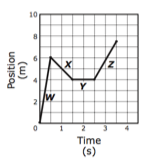
Motion sensors recorded the following data about a runner during a cross-country race. During which segment of the race did the runner have the greatest speed?
A) W
B) X
C) Y
D) Z
A) w
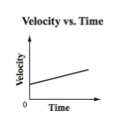
Which is the best description of the map below?
A) The car initially travels at a constant speed and then stops.
B) The car starts from rest and then travels at a constant speed.
C) The car starts from rest and then accelerates at a constant rate.
D) The car is initially moving and then accelerates at a constant rate.
D) The car is initially moving and then accelerates at a constant rate
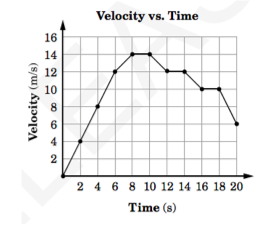
What is the average acceleration of the object during the first 4 seconds?
A) 1 m/s/s
B) 2 m/s/s
C) 4 m/s/s
D) 8 m/s/s
B) 2 m/s/s
Which compound is ionic?
A) CaCl2
B) N2O
C) HCl
D) SO2
A) CaCl2
Within Period 2 of the Periodic Table, as the atomic number increases, the atomic radius generally
A) decreases
B) increases
C) remains the same
When an atom loses an electron, the atom becomes an ion that is
A) positively charged and gains a small amount of mass
B) positively charged and loses a small amount of mass
C) negatively charged and gains a small amount of mass
D) negatively charged and loses a small amount of mass
B) positively charged and loses a small amount of mass
Chlorine atoms _________ when they form chemical compounds.
A) gain electrons
B) lose electrons
C) stay the same
A) gain electrons
Elements on the periodic table are identified and ordered by their ______
A) atomic number
B) atomic mass
C) number of electrons
D) number of protons
A) atomic number
Oxygen would have similar properties to
A) Sulfur
B) Nitrogen
C) Fluorine
D) Carbon
A) sulfur
Which atom has five valence electrons?
A) Germanium
B) Oxygen
C) Arsenic
D) Iodine
C) arsenic
Which element has two valence electrons?
A) Barium
B) Cesium
C) Aluminum
D) Sulfur
A) barium
Which of the following atoms has the greatest atomic radius?
A) Rb
B) Sr
C) P
D) S
A) Rb
Which pair of elements has the most physical and chemical properties in common?
A) Ge and Se
B) K and Kr
C) Al and Ga
D) Ba and Bi
C) Al and Ga
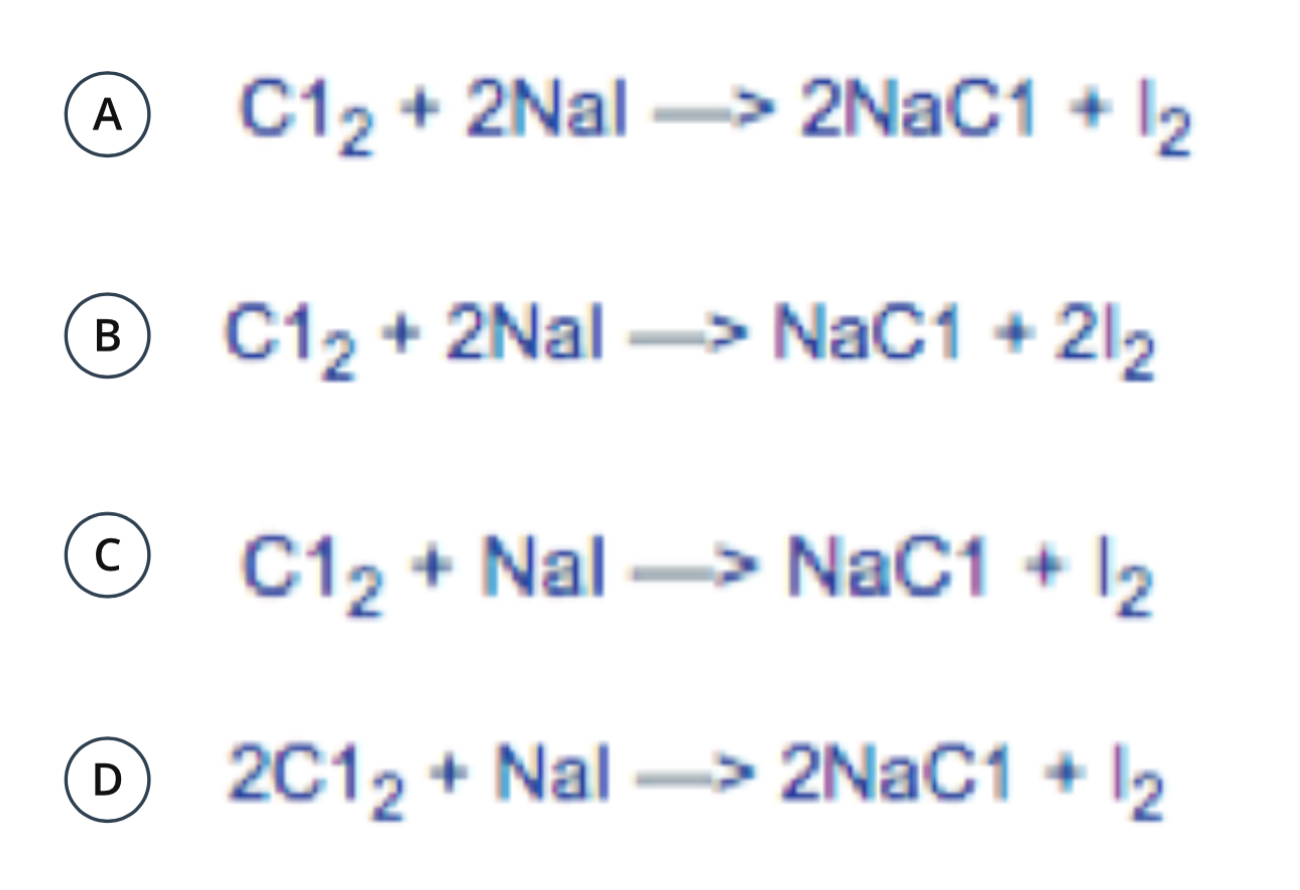
Which is the correctly balanced equation?
A
Which best represents how electrons are arrange in the energy levels of a carbon atom?
A) first energy level = 1 electron; second energy level = 5 electrons
B) first energy level = 2 electrons; second energy level = 4 electrons
C) first energy level = 3 electrons; second energy level = 3 electrons
D) first energy level = 4 electrons; second energy level = 2 electrons
B) first energy level = 2 electrons; second energy level = 4 electrons
Which statement best describes the atoms of elements that form compounds by covalent bonding?
A) they have a large difference in atomic mass
B) they are in the same period in the periodic table
C) they have a large difference in valence electron number
D) they share electrons between them
D) they share electrons between them
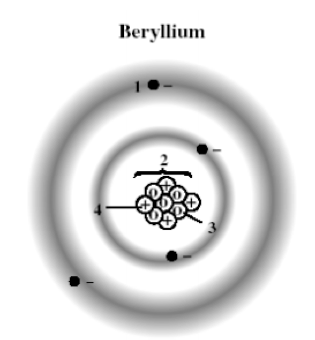
The following diagram shows a model of a beryllium atom. Which marked position represents a proton?
A) 1
B) 2
C) 3
D) 4
D) 4
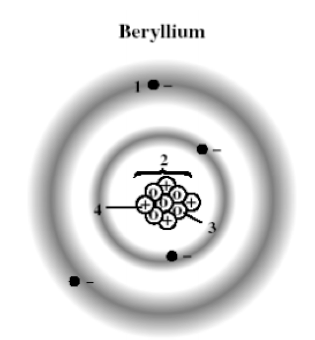
The following diagram shows a model of a beryllium atom. Which marked position represents an electron?
A) 1
B) 2
C) 3
D) 4
A) 1
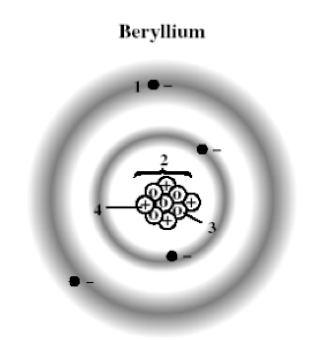
The following diagram shows a model of a beryllium atom. Which marked position represents a neutron?
A) 1
B) 2
C) 3
D) 4
C) 3
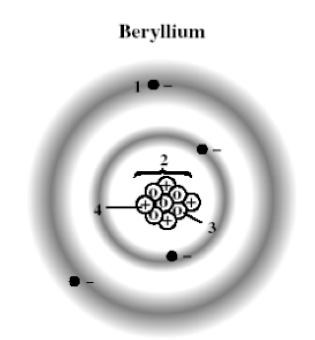
The following diagram shows a model of a beryllium atom. Which marked position represents the nucleus?
A) 1
B) 2
C) 3
D) 4
B) 2
The valence shell of a neutral atom loses two electrons. Which of the following ions might result?
A) O2-
B) K+
C) N3-
D) Mg2+
D) Mg2+
An atom has 29 protons, 29 electrons and 35 neutrons. What is the mass number of the atom?
A) 29
B) 35
C) 64
D) 93
C) 64
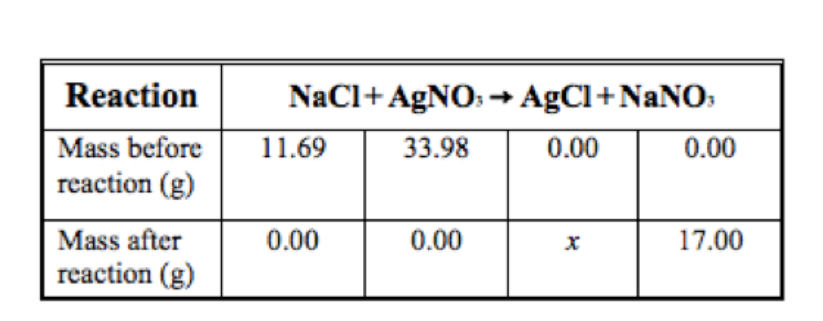
The table below shows the reaction of sodium chloride with silver nitrate and the masses of the compounds involved in the reaction. (Hint: Remember the law of conservation of mass)
How many grams of AgCl, x, are produced by the reaction?
A) 16.98
B) 28.67
C) 45.67
D) 62.67
B) 28.67
How many sig figs are in the following number?
2.090
4
Make the following calculation and record the appropriate number of sig figs.
17.10 • 102 =
1740
Make the following calculation and record the appropriate number of sig figs.
8,000. ÷ 60. =
130
How many significant figures are in the following value?
80.040
5
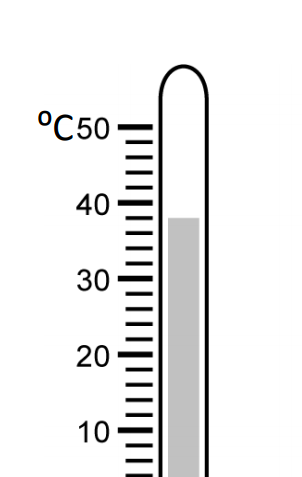
Read the instrument below and record its measurement to the appropriate number of significant figures.
38.0

Setup I: What is the correct measurement that should be recorded to the correct number of significant figures?
294
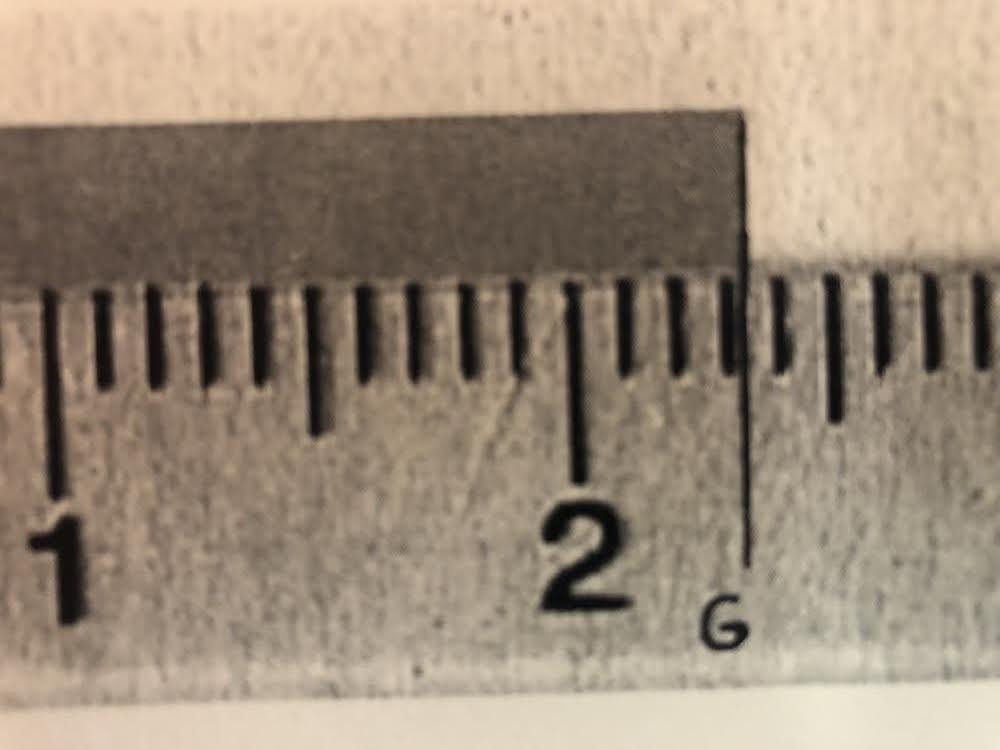
Setup G: What is the correct measurement that should be recorded to the correct number of significant figures?
2.31
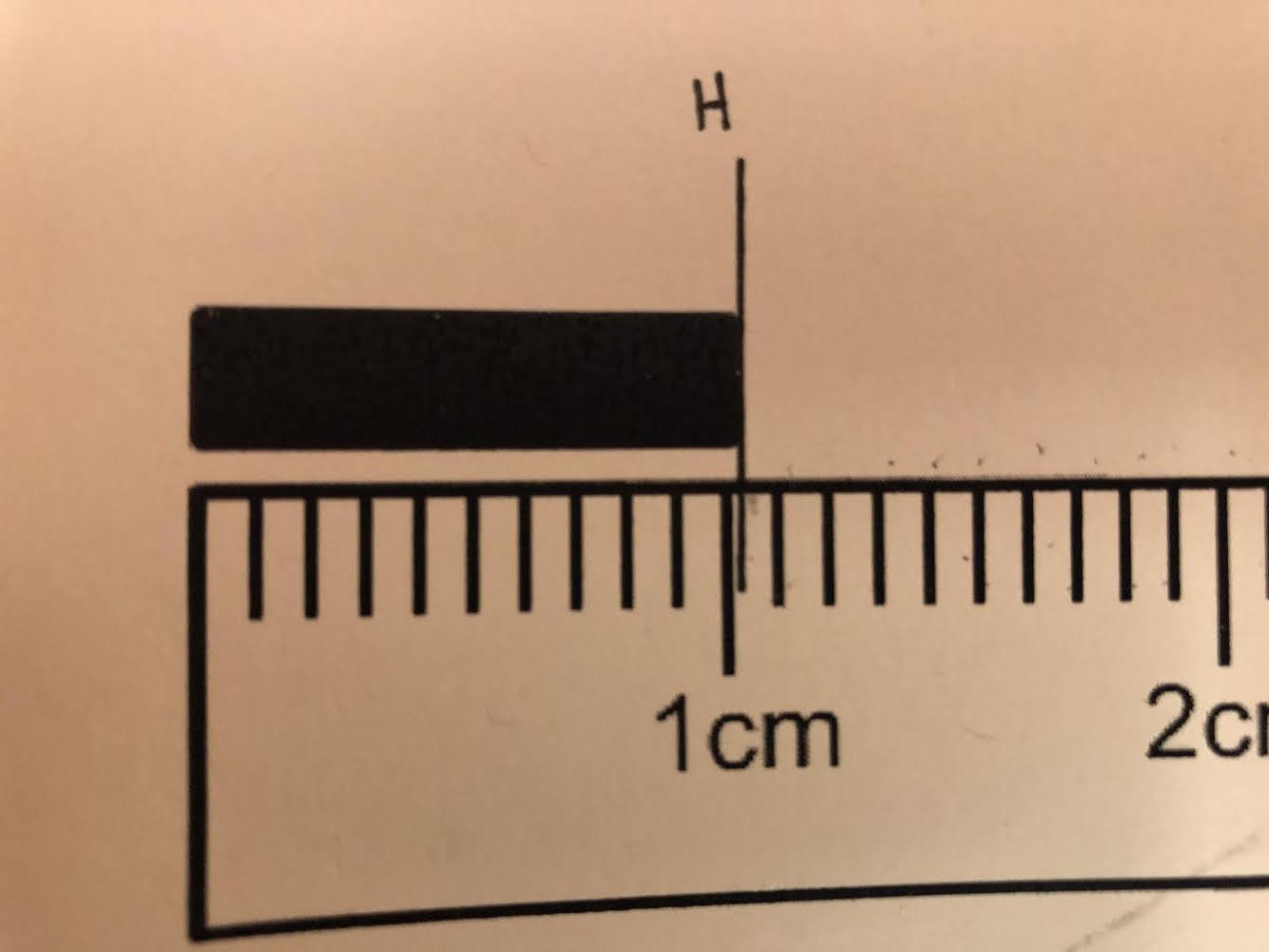
Setup H: What is the correct measurement that should be recorded to the correct number of significant figures?
1.01
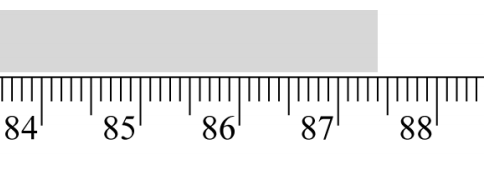
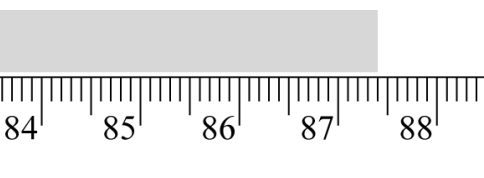
Read the instrument below and record its measurement to the appropriate number of significant figures.
87.40
Which measurement would be obtained in the setup pictured below?
A) 5.10 cm
B) 5 cm
C) 51 cm
D) 5.05 cm
E) 5.15 cm
A) 5.10
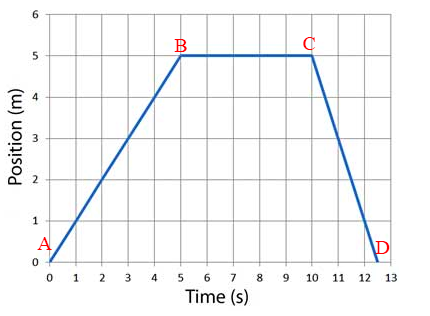
Which segment of the graph represents an object moving at a constant rate in the leftward direction?
A) Section A-B
B) Section B-C
C) Section C-D
D) None of the sections represent the motion described
C) section C-D
Atomic radius _____ as we move down the periodic table
A) increases
B) decreases
C) stay the same
A) increases
Atomic radius______as we move to the right of the periodic table
A) increases
B) decreases
C) stay the same
B) decreases
Which element on the periodic table has the highest electronegativity?
Fluorine
tendency of an atom to attract electrons toward itself.
Electronegativity
Outer electron shell of an atom
Valance shell
Bond that occurs between a metal and non metal when the metal gives up one or more electrons to the nonmetal.
Ionic bond
Bond that occurs between two nonmetal atoms when they share electrons.
Covalent bond
a number placed before a reactant in a chemical equation so that the number of atoms in the products on the right side of the equation are equal to the number of atoms in the reactants on the left side.
Coefficient
Difference in electronegativity between atoms is between 0.0 and 0.4
Nonpolar covalent
Difference in electronegativity between atoms is between 0.4 and 1.7.
Polar covalent
Difference in electronegativity between atoms is above 1.7
Ionic