Chemistry - Midterm 1
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
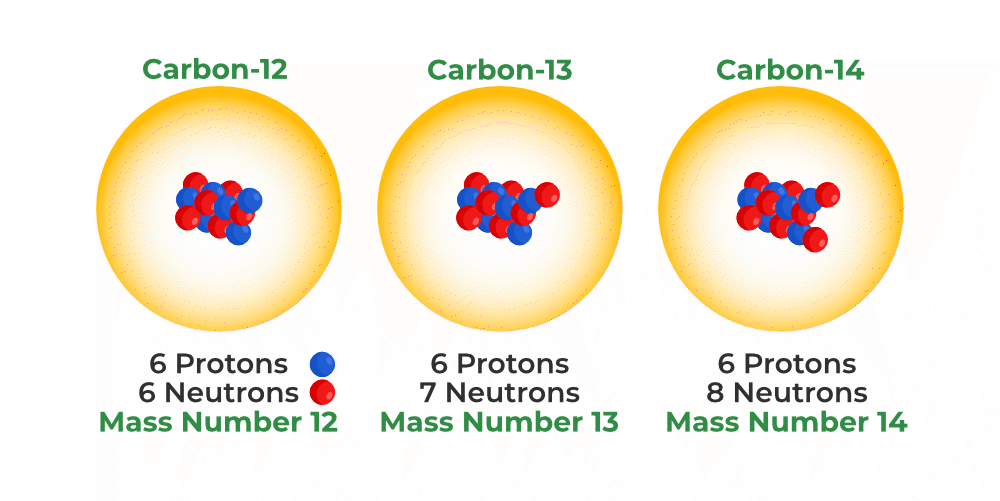
isotope
versions of a particular element that have different numbers of neutrons
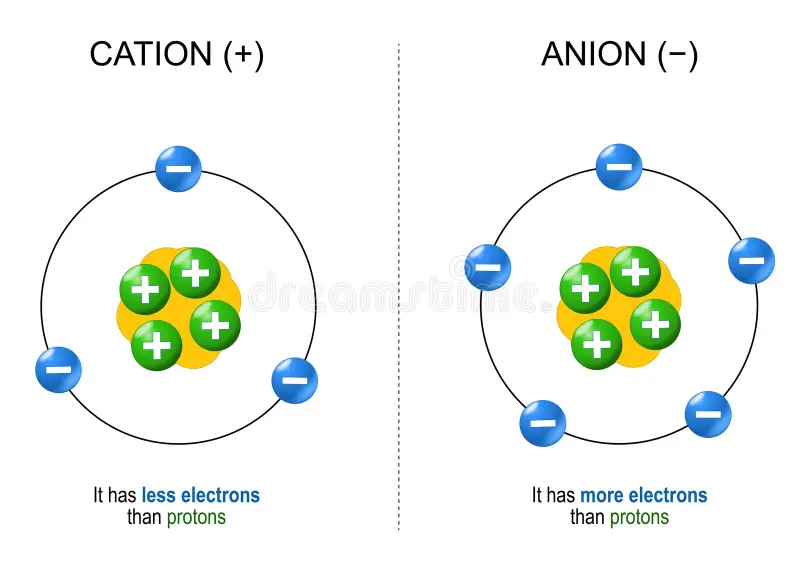
ion
atoms (or molecules) that have lost or gained electrons and have an electrical charge
density formula
d = m/v
moles formula with mass
moles = mass / molar mass
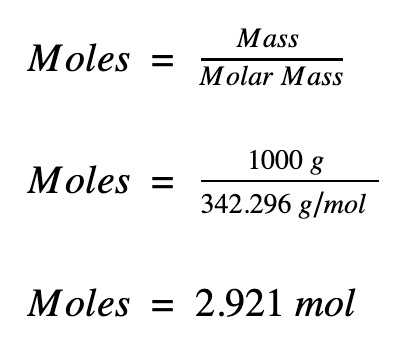
moles formula with atoms/particles
moles = (atoms/particles) / avogadros number
avogadros number = 6.022 × 10²³
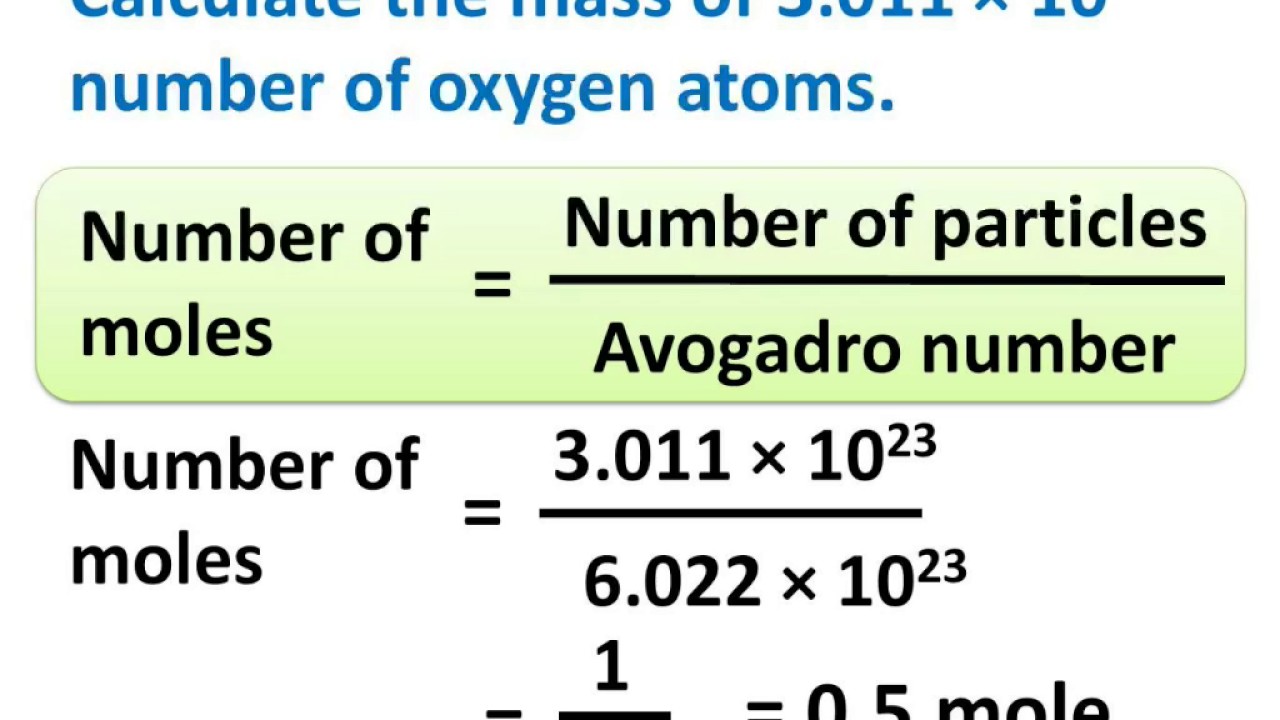
element
a pure substance made of only one type of atom.
Examples:
Gold (Au), Oxygen (O₂), Iron (Fe),
(titanium metal, a substance containing only titanium atoms)
(fluorine gas, a substance that contains only fluorine atoms)
compound
a pure substance made of two or more elements chemically bonded in a fixed ratio.
Examples:
Water (H₂O), Salt (NaCl), Carbon dioxide (CO₂).
phosphorus trichloride, a substance containing phosphorus and chlorine atoms in a fixed 1:3 ratio
carbon monoxide, a substance containing carbon and oxygen atoms in a fixed 1:1 ratio
homogeneous mixture
A uniform blend where components are evenly mixed (not chemically bonded).
Key Points:
Looks the same throughout (no visible parts).
Can be separated by physical methods (e.g., evaporation, distillation).
Examples:
Saltwater, Air
an alloy of copper and zinc
Earth’s atmosphere, which contains 78% nitrogen, 21% oxygen, and small amounts of other gases
heterogeneous mixture
a non-uniform mix where components remain distinct.
Key Points:
You can see the different parts (e.g., oil floating on water).
Easily separated by physical means (e.g., filtering, hand-picking).
Examples:
Salad dressing, Sand + iron filings, Granite
intensive property
properties that DO NOT DEPEND on the amount of matter
Examples:
boiling point
color
temperature
hardness
note: Density is an intensive property. Whether you have a small or large sample, the density of a given material will remain the same.
extensive property
properties that DO DEPEND on the amount of matter
Examples:
volume
mass
size
weight
length
chemical change (in a diagram)
a process where substances transform into new substances with different chemical properties
ex: Splitting a diatomic molecule, requires breaking chemical bonds, which is a chemical change
the relationship between energy, frequency, wavelength, and planck’s constant ((formulas))
e = hv
e = hc / λ ( this is also the formula for a single photon)
h = 6.626 × 10-34 (planck’s constant)
c = 3.00 × 108 (speed of light)
λ = wavelength
e = energy
ionic bond
transfer of electrons between atoms
covalent bond
sharing of electrons between atoms
chemical bond
the force that holds atoms together to create compounds and molecules
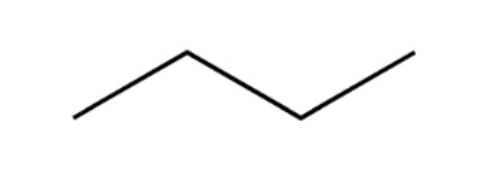
alkane
hydrocarbon containing only carbon-carbon SINGLE bonds
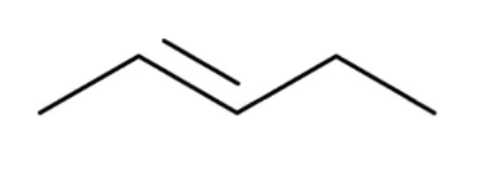
alkene
hydrocarbon containing one carbon-carbon DOUBLE bond
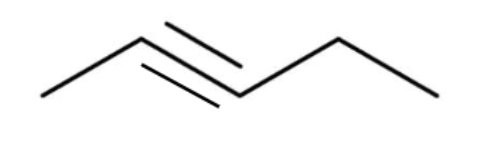
alkyne
a hydrocarbon containing one carbon-carbon TRIPLE bond
resonance structures
when more than one valid Lewis structure can be drawn for a molecule or ion
resonance structures are always the averages of all the structures
ionic solid
metal + nonmetal or contains polyatomic ions
metallic solid
contains metal only
network covalent solid
diamond, graphite, SiO2, SiC, Si, Ge
molecular covalent
nonmetal + nonmetal that is NOT network covalent