Biochem
1/138
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
139 Terms
Anabolism
reaction of synthesis (monomer → polymer) Requires energy
Metabolic pathways
a series of chemical reactions in a cell
Catabolism
Reaction of breakdown (polymer → monomer) Releases energy
Where do most metabolic reations takes place?
highly controlled aqueous environments (since 90% of a cell’s cytoplasm is water)
Factors affecting function and ability to react successfully in metabolic processes
Shape
Structure
Chirality (HL)
Factors that must be maintained to ensure optimal cell function in metabolic process
PH
Temperature
Concentration of components within the cell’s cytoplasm
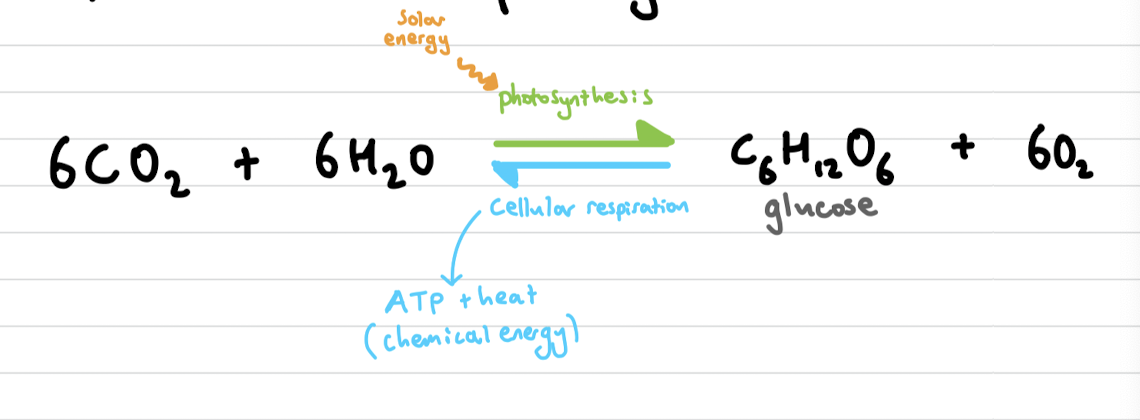
Photosynthesis
The synthesis of energy-rich molecules from carbon dioxide and water using light energy. (endothermic)
Respiration
A complex set of metabolic process providing energy for cells. (exothermic)
Aerobic vs Anaerobic
Aerobic: with oxygen
Anaerobic: in the absence of oxygen
write the equilibrium equation for photosynthesis and cellular respiration
Condensation reaction
produces water from H+ and OH-
Heat and acidic environment is often required
Anabolic
Hydrolysis
water breaking → H2O splits to H+ and OH-
Heat + Acid catalyst/enzymes required
Enzymes ensure it doesn’t reverse
Catabolic
Proteins
biopolymers of 2-amino acids, joined by amide links / peptide bonds
Polypeptide chains
more than 3 amino acids joined by peptide bonds
formation occurs in condensation reactions, reversed in hydrolysis
Isoelectric point
the pH at which a molecule carries no net electrical charge. It is the pH at which a molecule is electrically neutral.
What determines Isoelectric point
R groups. Acidic R groups favour acidic isoelectric point, vice versa.
Zwitterion
A molecule with both positive and negative charges, making it electrically neutral. It forms when an amino acid is at its isoelectric point.
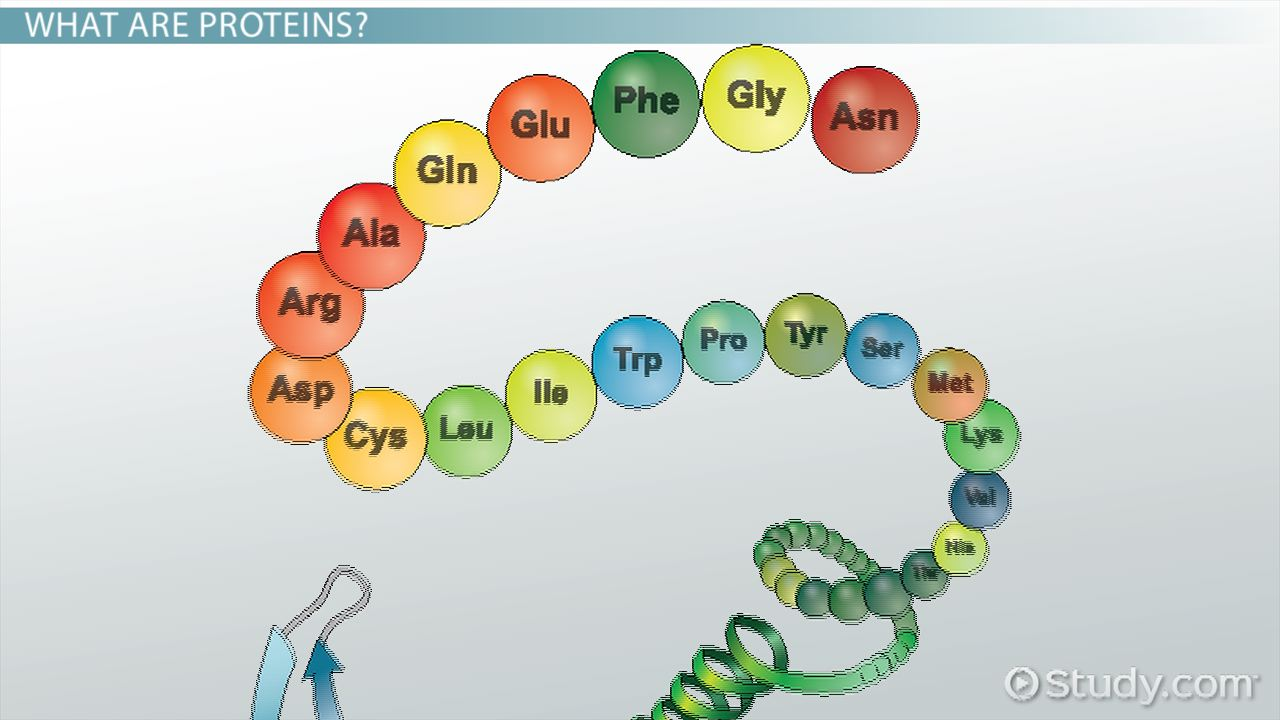
Primary protein structure
sequence of a chain of amino acids
bond: contains peptide bonds
example: polypeptide chains
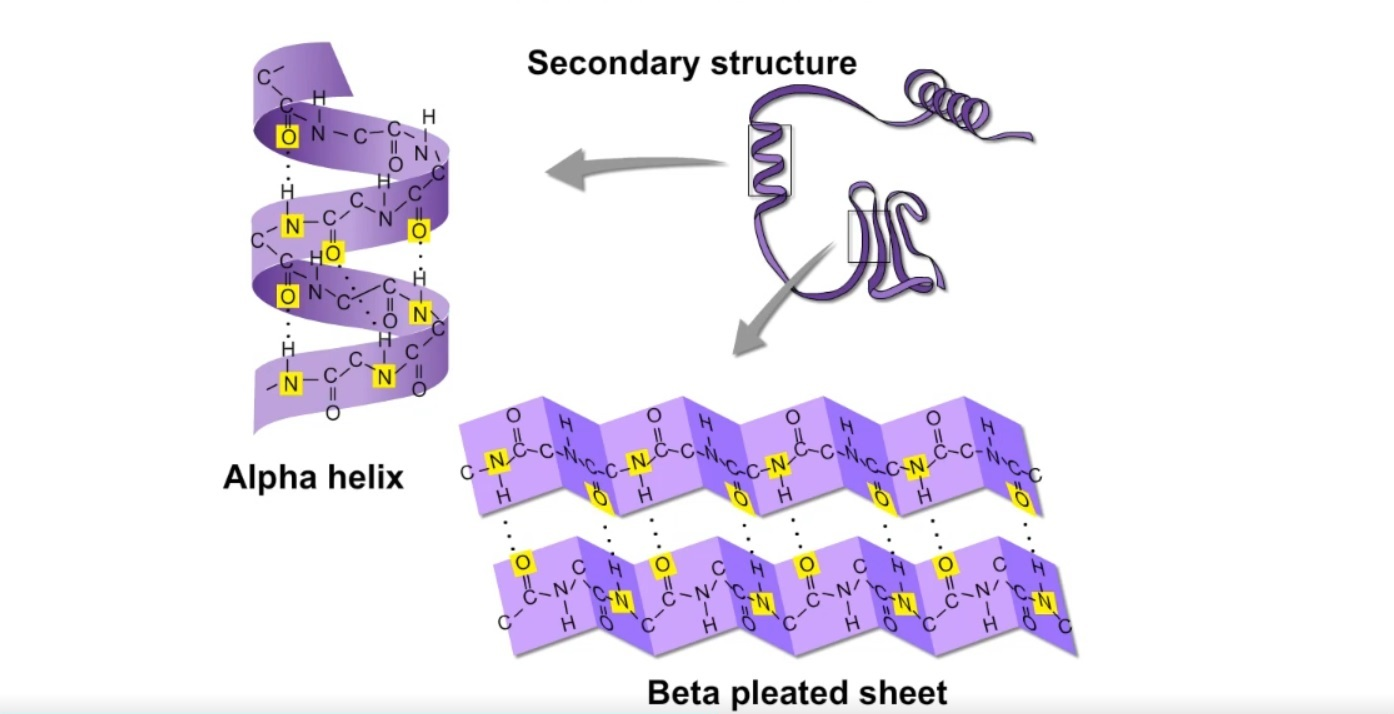
Secondary protein structure
amino acid folds into a repeating pattern due to H-bonds
bond: Hydrogen bonds between COOH and NH2
example: alpha helix and beta pleated sheets
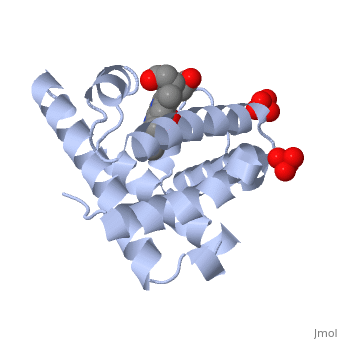
Tertiary protein structure
Three dimensional folding pattern of a protein due to side chain interactions
Bonds: between R-Groups
Hydrogen bonds: helps stabilise protein molecule
Disulfide bonds: strong covalent formed by oxidation of -SH groups in cystein side-chains
LDF: when two molecules are close to eachother they can appear
Ionic bonds: Weak electrostatic interactions
Example: myoblin or enzymes
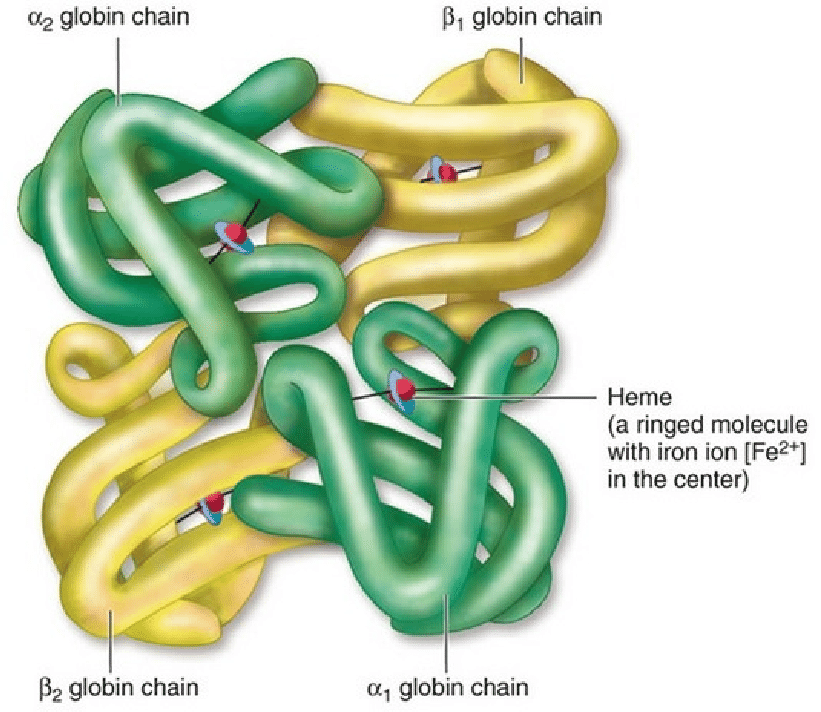
Quarternary protein structures
Proteins consisting of multiple polypeptide chains
Bond: between R-Groups
Example: Haemoglobin
What determines the role of a protein
3D shape determines its role in structural components (e.g. inside cells, tissues, organs, etc) or in metabolic processes
Globular proteins
spherical in shape
soluble in water due to polar R groups being on the outside, whilst non-polar R groups are on the inside
act as chemical messengers (hormones), catalysts (enzymes), and tranpsport molecules.
high temperatures denature the protein by weaking it’s IMF
Fibrous proteins / scleroproteins
Long linear bundles of polypeptide chains, held together by covalent bonds, SS bridge, or H-Bond.
insoluble in water due to exposed R groups being a mix of polar / non polar
Form the basis of structural elements (cells, tissues, etc.) in organisms
not as sensitive to high temperatures, as covalent bonds > IMF
Enzymes
“biological catalysts”
IB: most enzymes are proteins that act as catalysts by binding specifically to a substrate at the active site
usually aqueous as they are homogenous catalysts (same state as other reactants)
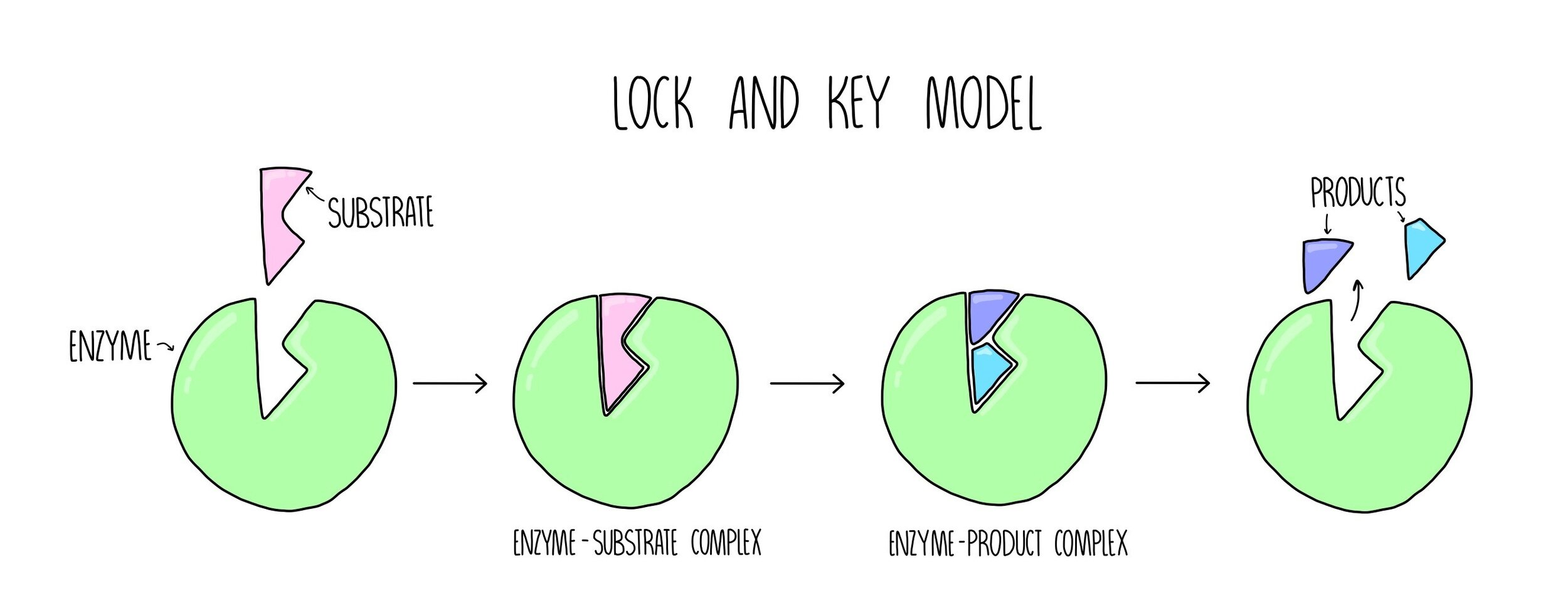
Lock and key model
An enzyme has a cleft in its surface, called the active site. The substrate molecule has a complimentary shape
An enzyme-substrate complex is temporarily formed. The R groups of the amino acids in the active site interact with the substrate.
The substrate is broken apart and the two product molecules leave the active site without damaging the enzyme molecule.
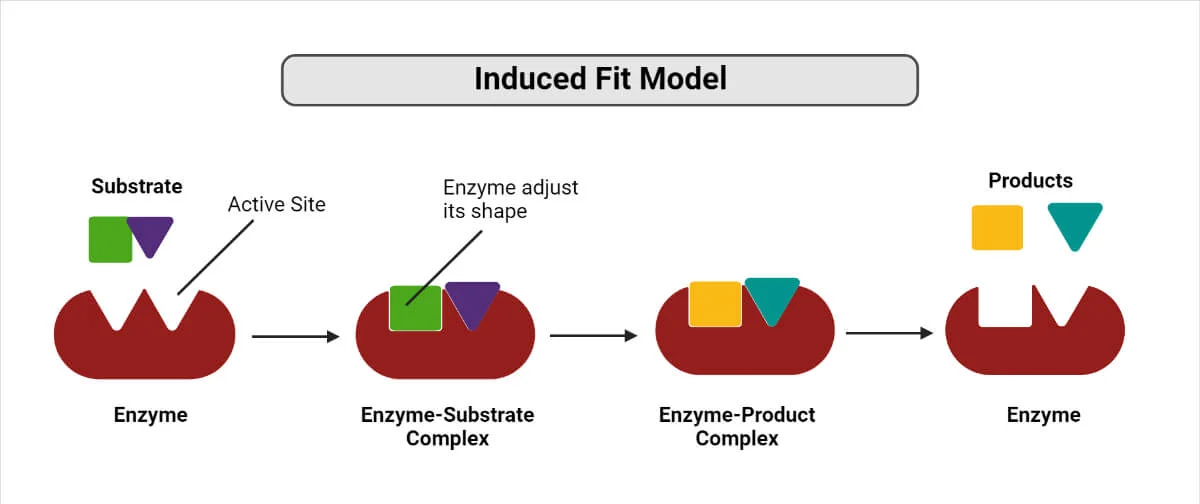
Induced fit model
An enzyme has a cleft in its surface, called the active site. The substrate molecule does not have a complimentary shape
The enzyme changes shape slightly as substrate binds
enzyme-substrate complex is temporarily formed. The R groups of the amino acids in the active site interact with the substrate.
The substrate is broken apart and the two product molecules leave the active site without damaging the enzyme molecule.
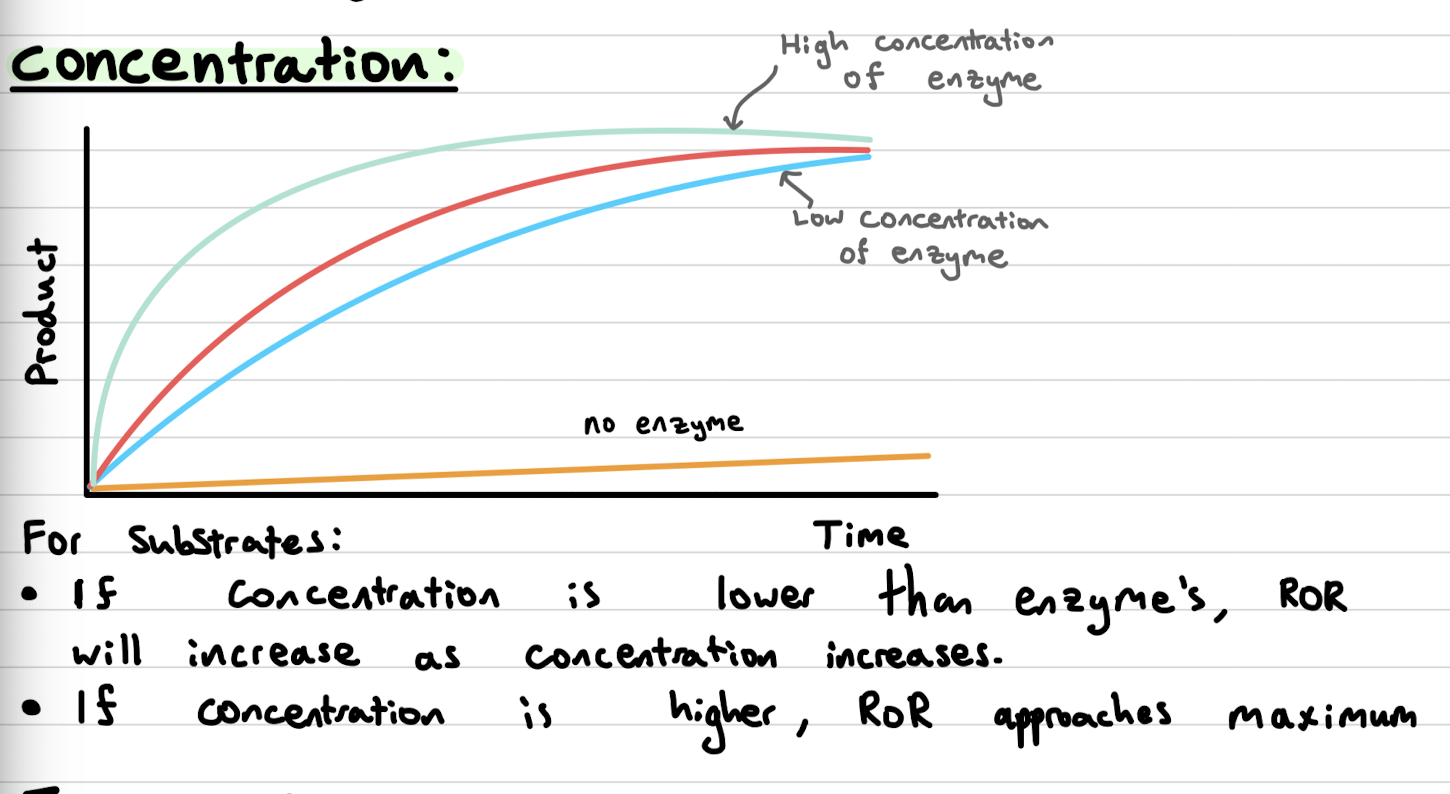
What factors affect enzymatic activity
Concentration
Temperature
pH
Heavy metal ions
How does concentration of enzymes/substrates affect enzymatic activity
How does temperature affect enzymatic activity
Enzymes are globular proteins, therefore heat can denature them by interupting IMF
Denatured (changed shape) enzyme cannot perform its function, since the active site has changed shape
Enzymes has a maximum rate of activity at a certain optimal temperature, which is why our bodies control temperature
How does pH affect enzymatic activity
changes to pH of aqueous environment can cause amino acid residues to gain or lose protons, therefore changing their shape and interactions
Enzymatic activity has an optimal pH level
Heavy metal ions and enzymes
some heavey metal ions can bind to an enzyme, causing a decrease in enzymatic activity
common ions like lead (II) and mercury (II) can bind to enzymes and cause negative health effects
bond is covalent, the binding is strong and permanent
How are proteins broken down into component amino acids for protein analysis
Hydrolysis reaction using conc. HCl
Describe steps of paper chromatography
Small sample of amino acid mixture spotted on the origin
Filter paper is supended in solvent with the origin above the solvent level
As solvent rises, amino acids will distribute themselves between 2 phases:
stationary phase - absorbs more strongly to stationary phase means its lower down
mobile phase - more soluble in mobile phase means it will go higher up
Once removed, sprayed with ninhydrin (organic dye / locating reagent)
Analysis can be conducted
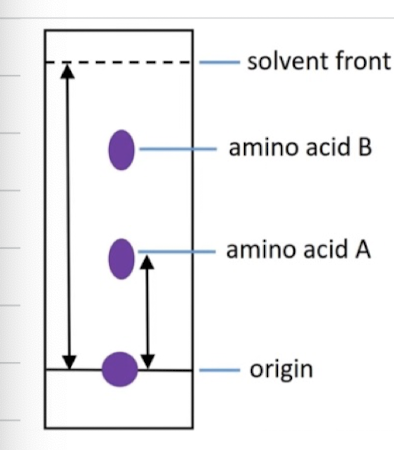
What is the retention factor and what does it do
Number between 0 and 1, given by equation above. Helps identify the amino acid, by comparing to known values
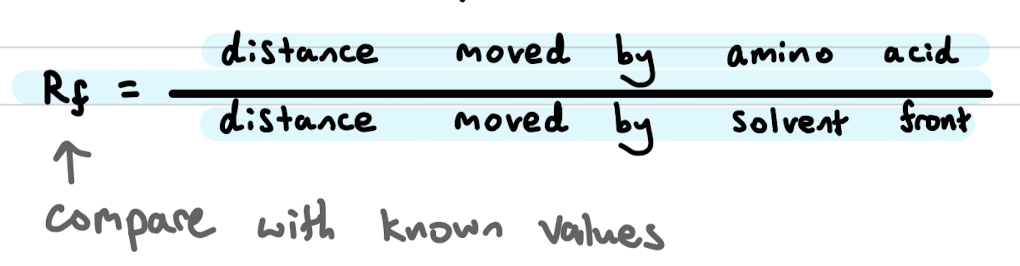
What is gel electrophoresis and describe steps
a method of seperation and analysis of biomacromolecules and their fragments based on their size and shapes
amino acid mixture is placed in the sample wells of the gel (usualy polyacrylamide)
A potential difference (voltage) is applied
amino acids with a positive charge in the buffer are attracted to the cathode, vice versa (smaller goes further also)
once seperated, sprayed with ninhydrin (organic dye)
identified by measuring distance travelled then comparing to known samples
What’s a lipid and what are the types and functions
Organic molecules with long hydrocarbon chains that are soluble in non-polar solvents.
Triglycerides - energy storage / thermal insulation
Phospholipids - components of cell membranes / electrical insulation in nerves
Steroids - hormones
involved in the transportations of fat soluble vitamins (A, D, E, and K)
why are fats better at energy storage gram for gram
Fats are more reduced than carbohydrates, therefore yield more energy when oxidised
Solubility and energy storage of carbohydrates and fats
Carbohydrates
Soluble in water - polar OH groups form H-bonds
therefore more rapidly transported in body, so it’s used for short term energy storage
Fats
Insoluble in water - as they have non-polar hydrocarbon chains
therefore slowly transported in the body, so it’s used for long term energy storage
Fatty acids
“building blocks of fat”
Carboxylic acids with a long hydrocarbon chain. Can be saturated, monounsaturated, or polyunsaturated.
Saturated fatty acids + melting point
called “fats”
109.5 degree bond angle (tetrahedral)
single C-C bonds
therefore no kinks due to double bond
therefore allows molecules to pack closely together
therefore stronger LDF
higher M.P
unsaturated fatty acids + Melting point
called “oils”
mono: singular C=C bond
poly: multiple C=C bond
120 degree bond angle (trigonal planar)
double bond causes kinks in the chain
molecules can’t pack closely
weaker LDF
lower M.P
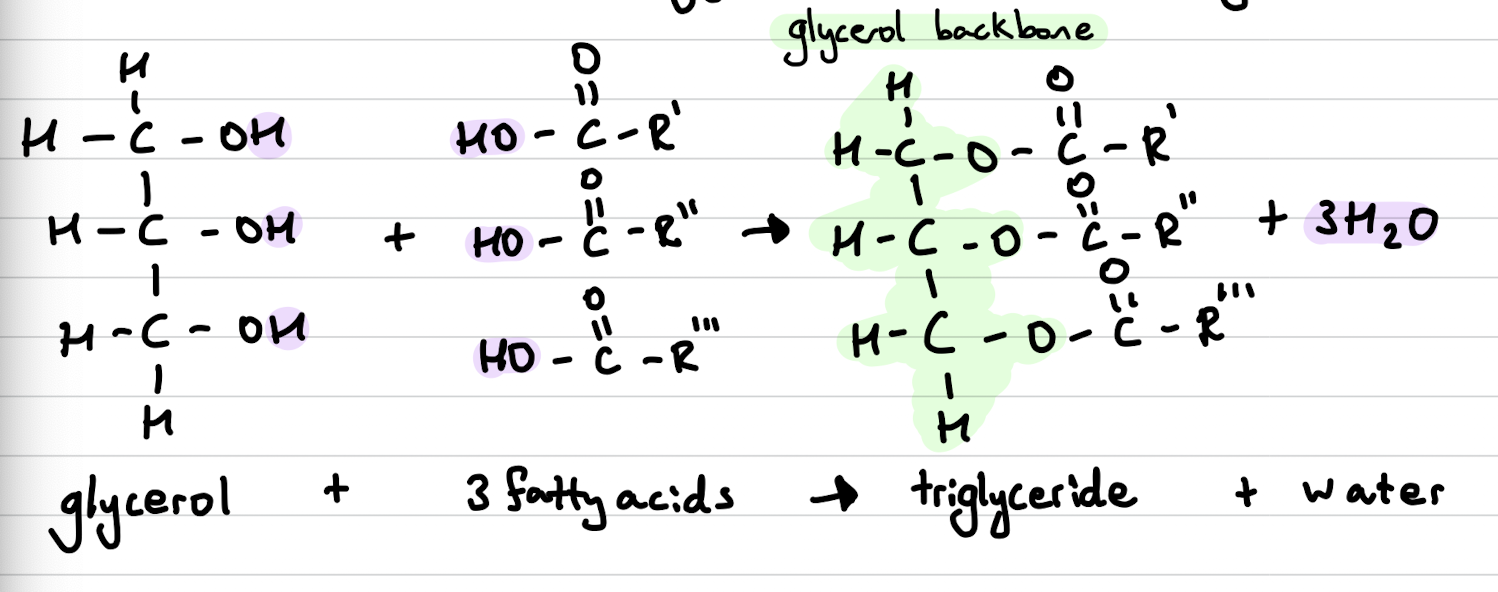
Triglycerides
Produced in a condensation reaction between a molecule of glycerol and 3 fatty acids.
Phospholipids
Derivatives of triglycerides, with non polar tails (fatty acids) and a polar head (phosphate group) on a glycerol backbone
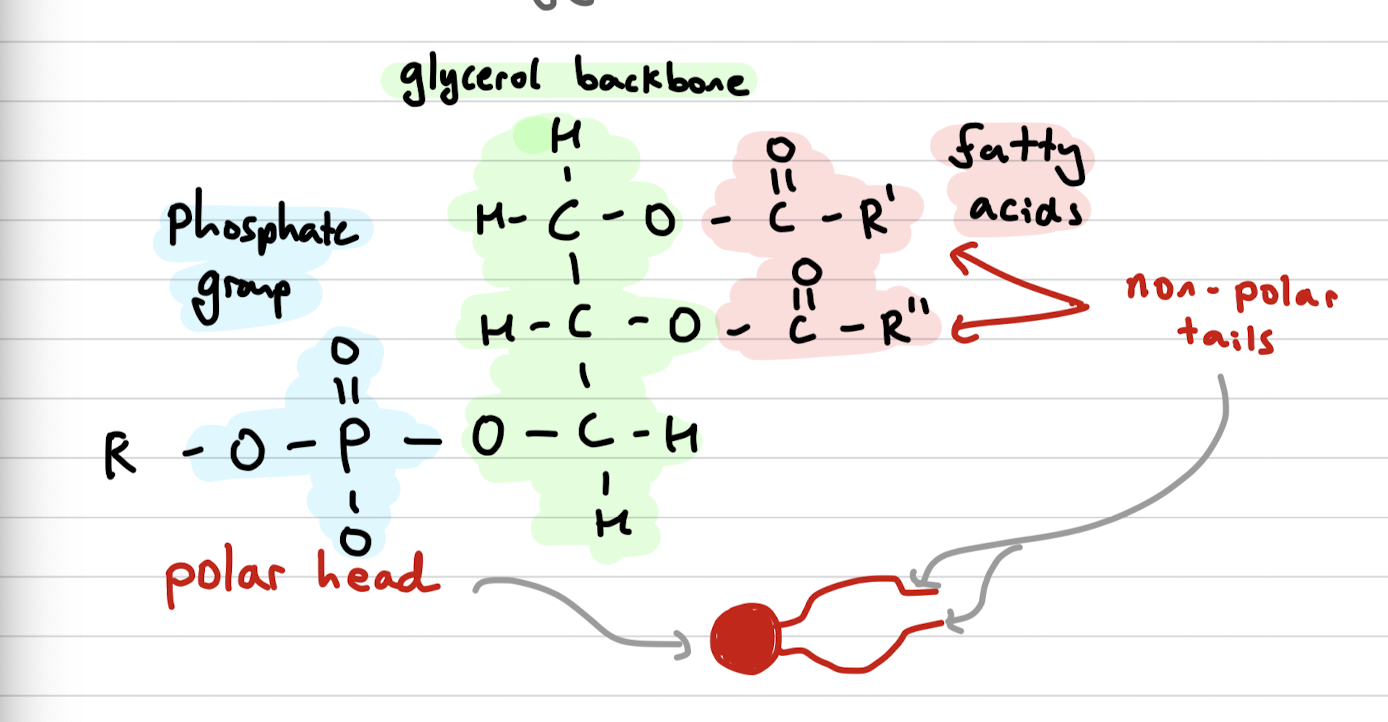
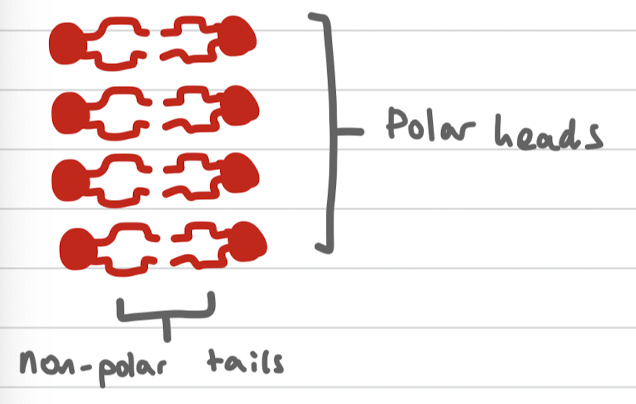
Phospholipid bilayer
A phospholipid bilayer is a double layer of phospholipid molecules that forms the basis of cell membranes. It consists of hydrophilic heads facing outward and hydrophobic tails facing inward, creating a barrier for the cell.
Steroids
A structure consisting of 4 fused hydrocarbon rings- 3 hexagons and 1 pentagon (steroidal backbone)
Uses and abuses of steroids
Uses:
Female steroid hormones in oral contraceptive or HRT
assist with rehab in building depleted muscles
Abuses:
Anabolic steroids used by athletes to get unfair advantage
Health effects of anabolic steroid abuse
On men:
infertility
breast development
shrinking of testicles
male-pattern baldness
become indian
On women:
decrease in breast size
decrease in body fat
deepening of the voice
excessive body hair
General:
Heart attacks and liver cancer
Cholesterol
has steroid backbone (3 cyclohexane, 1 cyclopentane)
transported around by lipoproteins -LDL + HDL
Cholesterol is a waxy substance found in your blood. Your body needs cholesterol to build healthy cells, but high levels of cholesterol can increase your risk of heart disease. With high cholesterol, you can develop fatty deposits in your blood vessels.
LDL (Low density lipoprotein)
Transports cholesterol to the arteries where it leads to the thickening of the walls of the arteries (atherosclerosis)
Main source: saturated fats; lauric acid, myristic acid and palmitic acid
Atherosclerosis
narrowing of artereries because lipids clog them
HDL (High density lipoprotein)
removes cholesterol from the walls of arteries
How to calculate iodine number
don’t do it the bullshit way. M(fat) is molar mass of fat
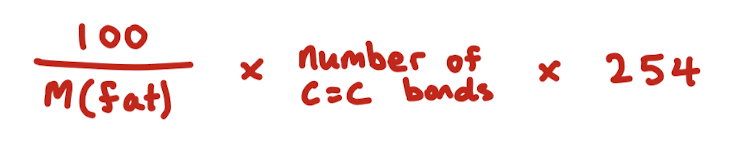
What does the iodine number tell us
Helps determine the unsaturation of a fat
What hydrolises fats and oils
lipase
types of hydrolysis of lipids
Acid hydrolysis
Hydrolysed in a hot aqueous solution of strong acid
triglyceride + water →(H^+ and heat)→ glycerol + 3 fatty acids
Alkaline hydrolysis
Produces salts of fatty acids (soaponification)
triglyceride + base → glycerol + soap
Phospholipids hydrolysis
occurs in the human body
phospholipid + water → glycerol + 2 fatty acids + phosphate group
Rancid
Disagreeable smell, texture, or appearance; Nick wang
Hydrolytic Rancidity:
Triglyceride + water → glycerol + 3 fatty acids
occurs quicker in heat and moisture, catalysed by the enzyme lipase
smell is due to release of fatty acids, can be reduced by refrigeration
sites of reactivity: ester linkages in triglycerides
Oxidative rancidity (auto-oxidation)
oxygen added across a C=C bond
catalyzed by light or enzymes and metal ions
occurs in highly unsaturated fatty acids
can be controlled with anti-oxidants
sites of reactivity: C=C unsaturated bonds
Monosaccharides empirical formula
CH2O
Haworth projections
3D Diagrams
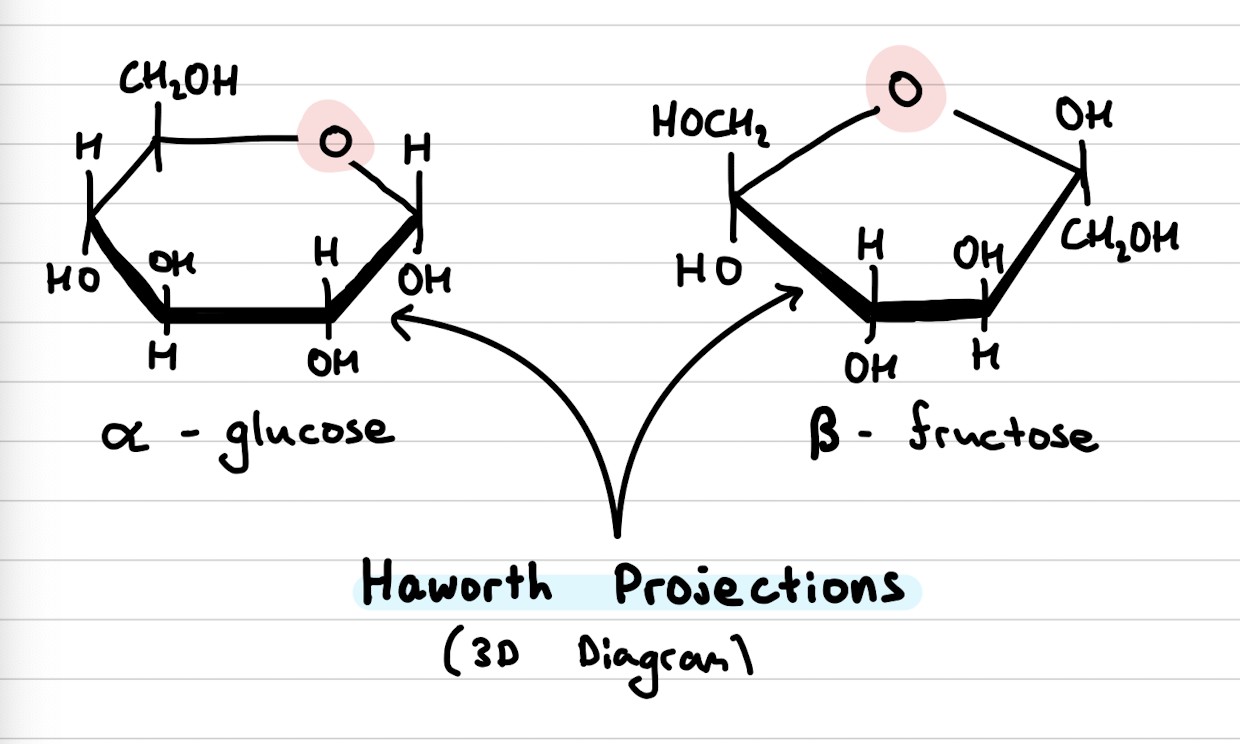
Monosaccharides
Simplest carbohydrate - cannot be hydrolysed to a smaller carbohydrate
one carbonyl group (-C=O) and two or more Hydroxyl groups (-OH)
in aqueous solutions, straight chain sugars form ring structures with an ether linkage (the O)
Disaccharides
Carbohydrate formed from two monosaccharides bonded by glycosidic links in condensation reactions
soluble molecules that can be hydrolysed into monosaccharides by acid hydrolysis or enzyme catalysed reactions
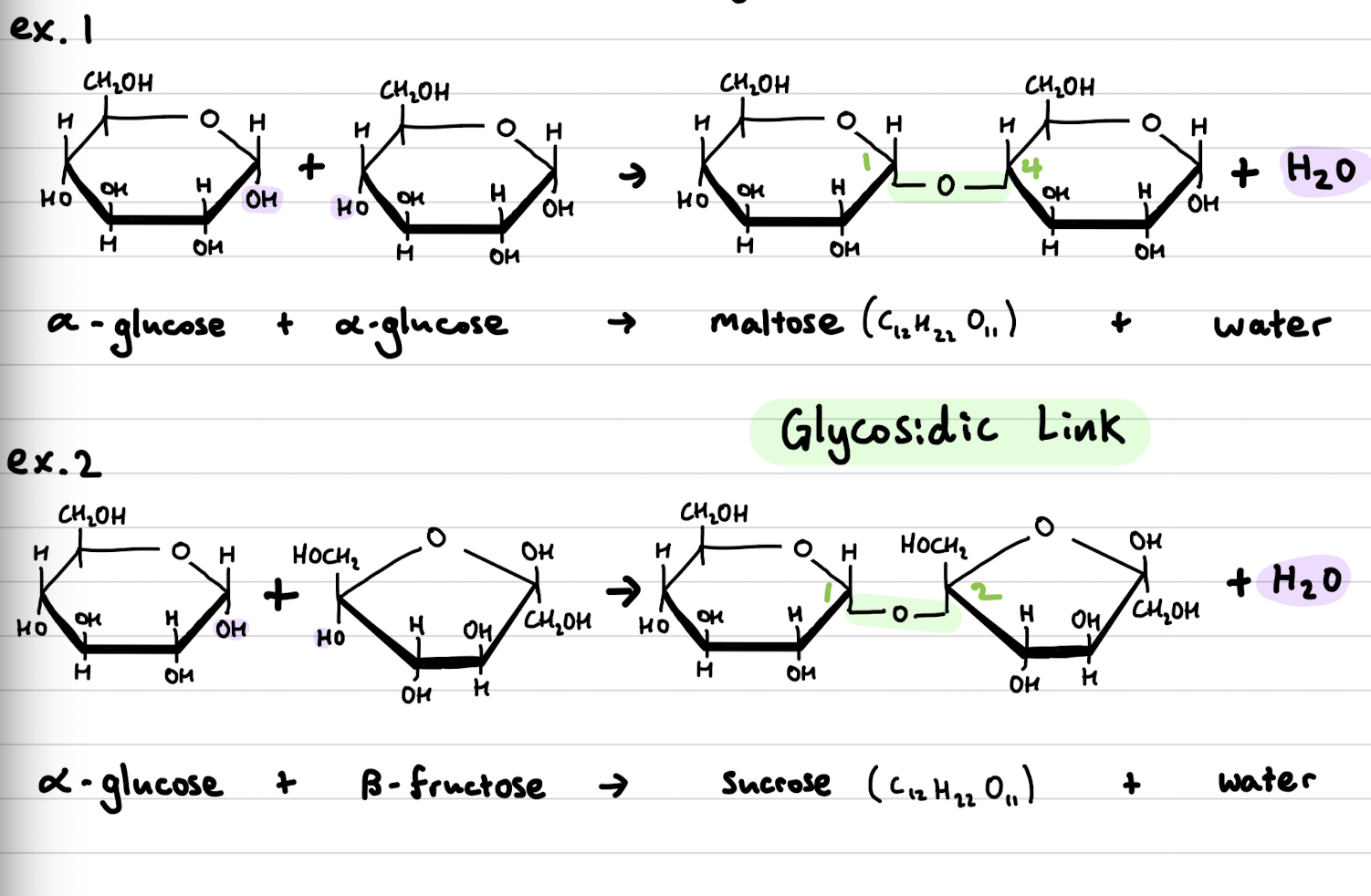
Disaccharides general formula
Cx(H2O)y
Polysaccharides
long chain of monosaccharides bonded by glycosidic links
used for energy storage:
starch: plants
glycogen: human
cellulose: structural material in plants and a dietary fibre for a balanced diet
Vitamins
organic micro-nutrients (substances needed in the body in amounts of <0.005% of body mass)
Not synthesised in body (except vitamin D), so must be obtained in diet
solubility can predict structure
Fat soluble vitamins
Vitamin A and D
non-polar hydrogen ring
one OH group, however non-polar part dominates
D: synthesised by sunlight on skin
A: important for low light vision (eat carrots)
Water soluble vitamins
Vitamin C
many polar OH groups from H-bond with H2O
high solubility in water means it’s not retained in the body for long
C=C bonds & OH groups are readily oxidised (by air or light)
fridge slows down oxidation
water soluble vitamins are sensitive to heat
Vitamin deficiencies causes
lack of distribution of global resources
depletion of nutrients in soil
lack of education about balanced diets
over-processing of foods
Vitamin deficiencies solutions
fortifying staple foods with vitamins
taking nutritional supplements
genetically modifying foods to increase vitamin content
educating people about balanced diets
Xenobiotics
Chemical substances found within an organism that are not naturally produced by or expected to be present within that organism
xeno - strange; alien
biotics - consists of living organisms
Antibiotics + sewage treatment
antibiotics are xenobiotics in animals
certain bacteria can become resistant to antibiotics
sewage treatment plants can promote the spread of antibiotic resistance between bacteria
water discharge into lakes and rivers from sewage treatment plants can contain significant concentrations of the genes that make bacteria antibiotic resistant
Polychlorinated biphenyl’s (PCB’s) and Dioxins and the environment
Toxic chemicals that persist in the environment
accumulate in body due to chemical stability
long-term exposure causes adverse health effects; possibly carcinogenic (cancer causing)
Biomagnification
The increase in concentration of a xenobiotic in a food chain
Example of DDT biomagnification
phytoplankton < zoo plankton < small fish < large fish < fish eating birds (in PPM of DDT).
DDT is readily soluble in fat and does not break down, so it accumulates in fatty tissue
birds of prey suffered a decline in numbers due to the toxic effects of DDT, it has now been banned in many countries
Biodegradability
Biodegradable (compostable) plastics can be consumed or broken down by bacteria or other living organisms
The role of starch in biodegradability
PLA is a biodegradable plastic derived from corn starch
the break down of starch-based plastics produces CO2 and H2O (aerobic conditions)
Bioplastics can be broken down in hydrolysis reactions due to ester linkages or glycosidic links (requires heat and moisture)
when some biodegradable plastics decompose, they produce methane gas (anaerobic conditions)
note: while they produce CO2 they are carbon neutral due to the CO2 they save in production
Host guest complex + bonds
Host guess complexes are composed of two or more molecules or ions that are held together through non-covalent bonding
Non covalent bonds:
H-Bonds
LDF
Dipole Dipole
Ionic
Hydrophobic interactions
host guest chemistry
Host guest chemistry involves the creation of synthetic host molecules that mimc some of the actions performed by enzymes in cels, by selectively binding to specific guest species
Applications of host guest chemistry
removal of xenobiotics in the environment
e.g. host-guest chemistry can remove Cs-137, which is radioactive from the environment
Vmax
Maximum rate of reaction where all active sites are bound to substrate (enzyme is saturated)
Km definition and meaning
The substrate concentration which is equal to half its maximum value
High Km: high concentration of substrate must be present to saturate the enzyme
Low Km: only a small amount of substrate is needed to saturate the enzyme
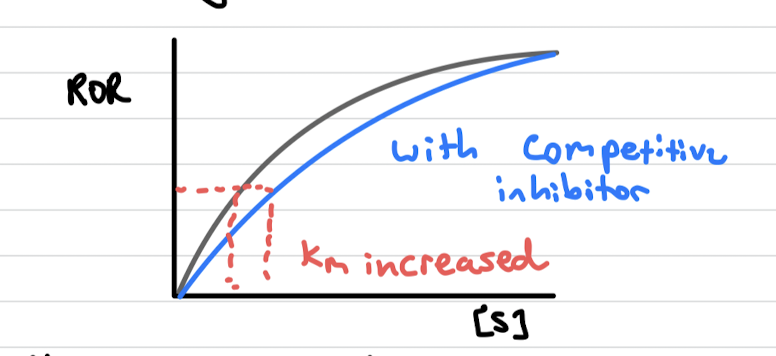
Competitive inhibitor
binds at the active site of the enzyme
chemical structure is similar to the substrate
once binded they do not form products, hence blocking the active site
increasing substrate concentration reduces extent of inhibition
Km increases and vmax stays the same
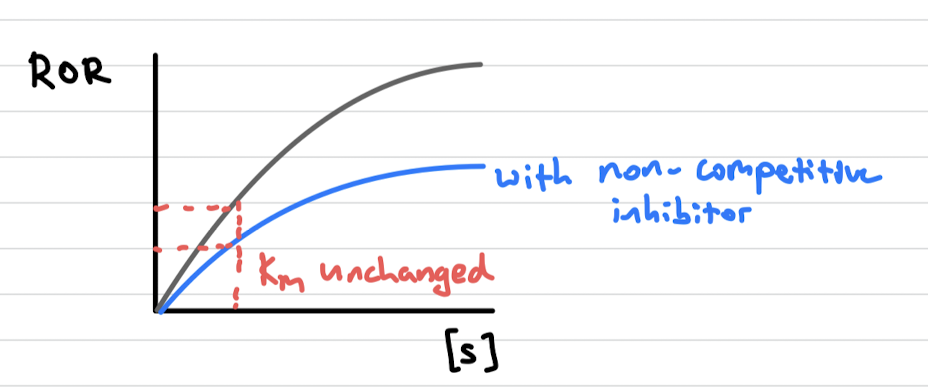
non-competitive inhibitors (2 points + graph)
binds at an allosteric site (away from active site)
changes protein’s conformation which alters shape of active site
km stays same and vmax decreases
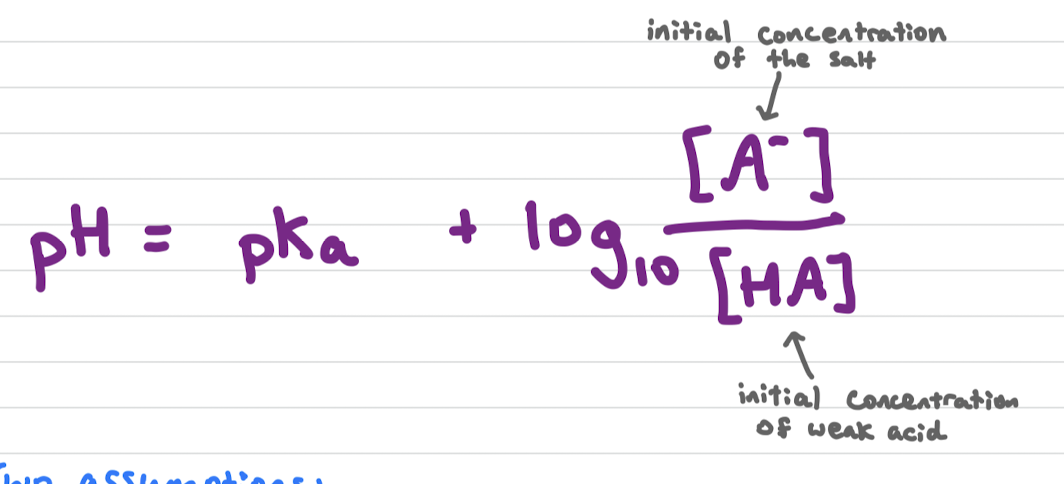
pH of buffer solution
initial concentration of the salt and initial concentration of weak acid
Assumptions of pH of buffer solution
weak acid partialy dissociates, therefore initial concentration of acid = equilibirium concentration
salt completely dissociates in solution, therefore initial concentration of salt = equilibirum concentration of the anion
Beer-Lambert Law
used t0 determine the concentration of a protein in solution from a calibration curve
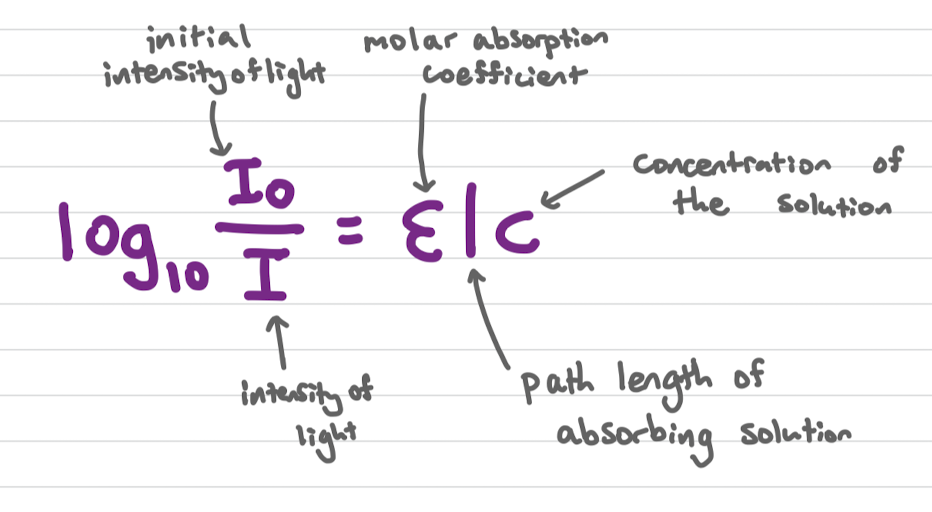
calibration curve
Using a range of solutions with known concentrations and measuring the absorbance with a spectrophotometer
in calibration curves absorbance is proportional to:
concentration (assuming fixed wavelength)
UV-Vis spectroscopy
Used in protein assays to measure the concentration of a protein in a sample
How does a spectrophotometer work
monochromatic light is incident on a protein sample with intensity i naught and hits the detector with intensity i
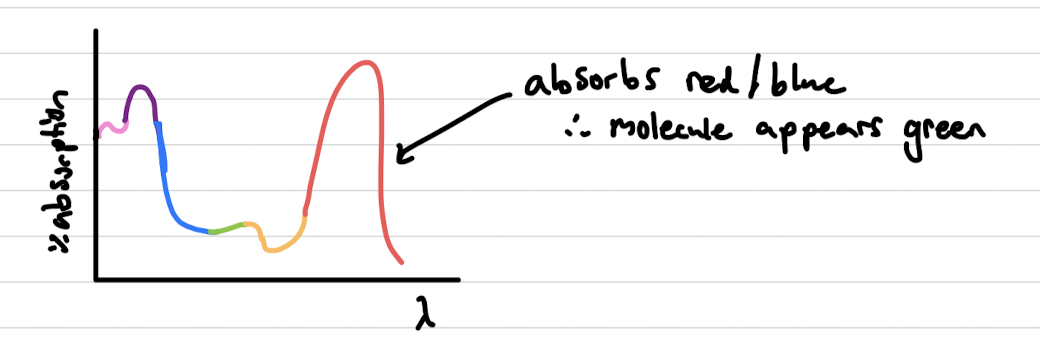
Absorption spectrum
least absorbed wavelength is emitted
Nucleotide
Phosphate group, pentose sugar and nitrogenous base (adenine)
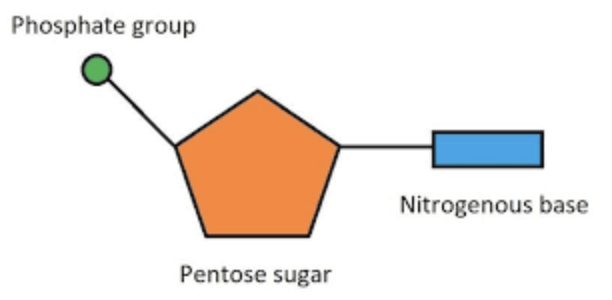
Types of bonds between constituent parts of nucleotide or between nucleotides
phosphodiester bond in condensation reaction
DNA
Stores genetic information
Double helix
Sugar phosphate backbones on outside, nitrogenous bases on inside holding the two strands together via Hydrogen-bonding
Deoxyribose is the pentose sugar
RNA
expresses the stored information (unzips DNA by breaking H-bonds)
single-stranded polynucleotide
sugar phosphate backbone
ribose is pentose sugar
Base pairing
A/T (2 H-bonds) or G/C (3 H-bonds) in DNA
DNA A / mRNA U
DNA T / mRNA A
Transcription
The process in which the information required to synthesise proteins is passed from DNA to mRNA. A segment of DNA is copied into mRNA by the enzyme RNA polymerase.
Translation
newly formed mRNA leaves the nucleus for the ribosome, producing a polypeptide chain
Codon
Triplet of nucelotides, represents one amino acid