midterm study guide (units #4-6)
UNIT 4 ✰
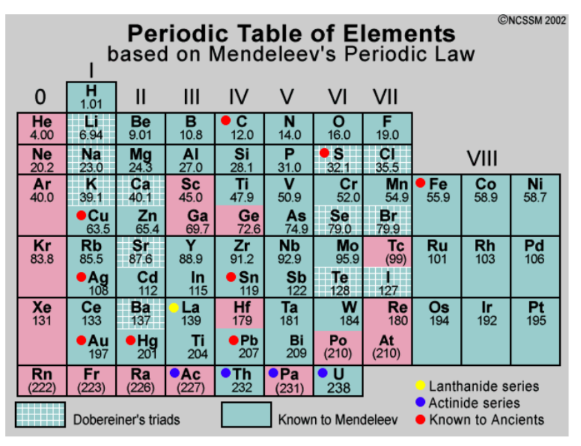
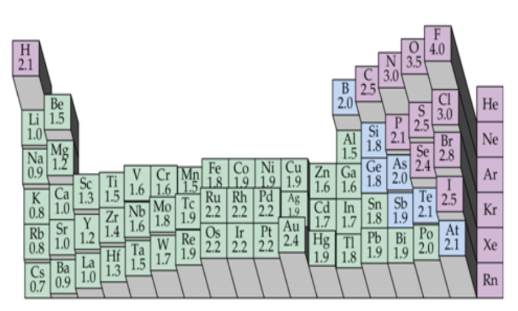
^^periodic table:^^
mendeleev:
not the first to organize elements by properties or into a list
- he was the first to use the properties to predict unknown elements
- order elements by increasing mass
- formulated the periodic law: the elements in the periodic table show patterns of characteristics (shown in columns)
moseley:
formulated moseley’s law
- this concretely established atomic number as the number of protons in an element
- before it, atomic number was just the order that an element was found on the periodic table
periodic table:
a. period: horizontal rows
(# energy levels/shells)
b. group: vertical columns/families
(elements with similar chemical properties)
^^types of elements:^^
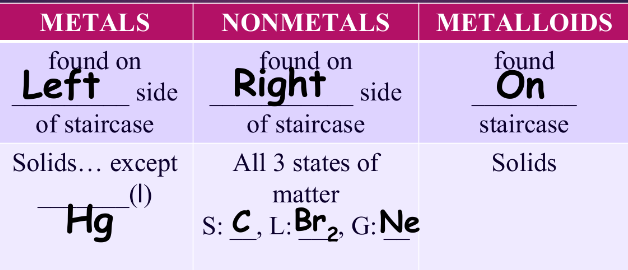
properties of metals:
- solids @ room temp. (EXCEPT Hg(l))
- shiny, has luster
- ^^malleable & ductile^^
- ^^high^^ melting points and boiling points
- ^^good conductors^^ of heat & electricity
- metallic bonds
properties of nonmetals:
- can be s, l, or g @ room temp.
- solids: B, liquid: Br2, gases: He
- ^^brittle, bull, lack luster^^
- poor conductors of heat & electricity
-^^covalent bonds^^
^^allotropes^^: different molecular forms of the same elements (diff. properties too)
ex.:
oxygen - O2 (atmospheric oxygen) & O3 (ozone)
carbon - diamond & graphite
properties of metalloids:
- solids @ room temp.
- semi-conductors
- varying properties (metallic/non-metallic)
valence electrons: the number of electrons in the
^^OUTERMOST^^ energy level of an atom
^^groups:^^
group 1 - ^^alkali metals^^
- DOES NOT INCLUDE HYDROGEN
- 1 electron in the outermost energy level (this gets lost to form 1+ ions)
- contains the most active metals—cannot be found in natural state
- as you go down the group, reactivity increases
group 2 - ^^alkaline earth metals^^
- 2 electrons in the outermost energy level (this gets lost to form 2+ ions)
- as you go down the group, reactivity increases
group 3-12 - ^^transition metals^^
- they transition from 8 electrons per shell to 18 then to 32
- most notably, they form colored compounds because they have multiple oxidation states
- technically includes the two bottom rows, which are called the lanthanides and actinides, respectively (also known as the rare earth metals)
- everything above element 92 is manmade
group 13-16 - ^^metalloids included^^
metalloid containing groups (most contain a mix of nonmetals, metalloids, and metals, with the elements becoming more metallic as you go down
group 17 - ^^halogens/”salt formers”^^- the most active nonmetals- these tend to gain electrons to form a 1- charge, some do behave differently, however (typically have 7 valence electrons, just missing 1)- all of these are diatomic
high level chemistry note:
we pretty much have no idea about astatine: rarest element, lasts for like a day, will vaporize itself
group 18 - ^^noble gases^^
- they pretty much do not react at all
- monoatomic
- full valence electron shells (this is why they are unreactive gases)
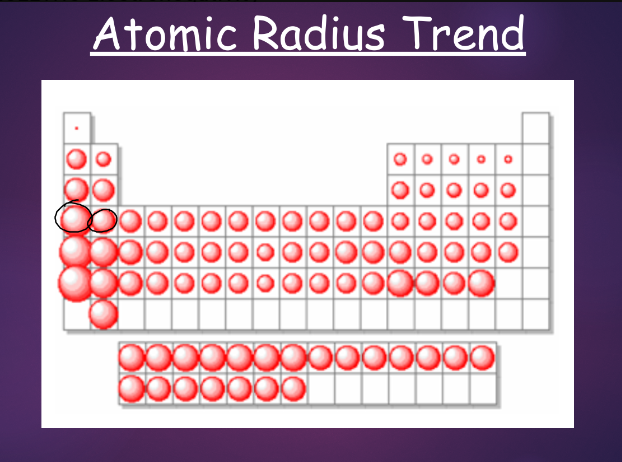
^^atomic radius:^^
recall: most of the atom is empty space
- we can still measure the size of an atom using a measure called atomic radius—this is defined as half the distance between two adjacent atoms in a crystal or half the distance between nuclei of identical atoms that are bonded together
- atomic radius increases as they go down a group—more inner electrons = less attraction to the nucleus, and more energy levels = further away from nucleus
- atomic radius decreases as you go across a period—increased number of protons felt more than increased number of electrons; electrons are attracted to nucleus
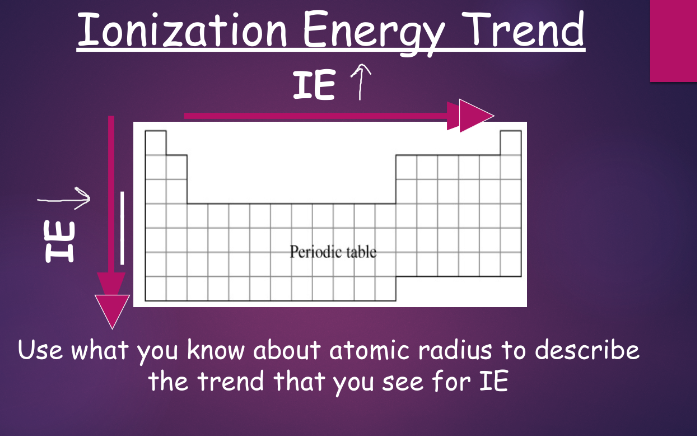
^^ionization energy:^^
- ions are formed when atoms gain or lose electrons
- electrons can gain energy to jump to higher energy levels
to lose an electron, one of an atom’s electrons must gain enough energy to escape from an atom (we call this ionization energy)
- table s also shows ionization energy for listed elements, with units of kJ/mol
^^higher ionization energy^^ = an atom is more likely to keep its electrons
as you go down a group, ionization energy decreases
as you go across a period, ionization energy increases
electronegativity: an atom’s ability to attract/gain electrons
- scales from 0-4
important reference point: F has the highest electronegativity
nonmetals generally have higher values—they tend to gain electrons
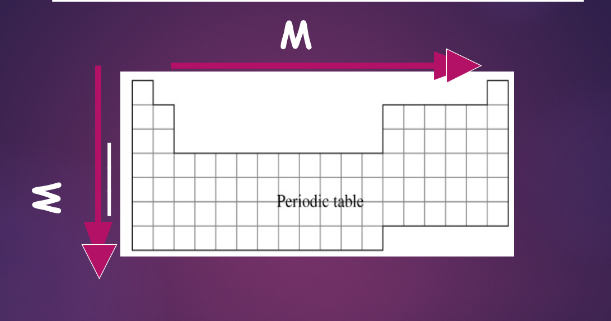
^^metallic character trend:^^
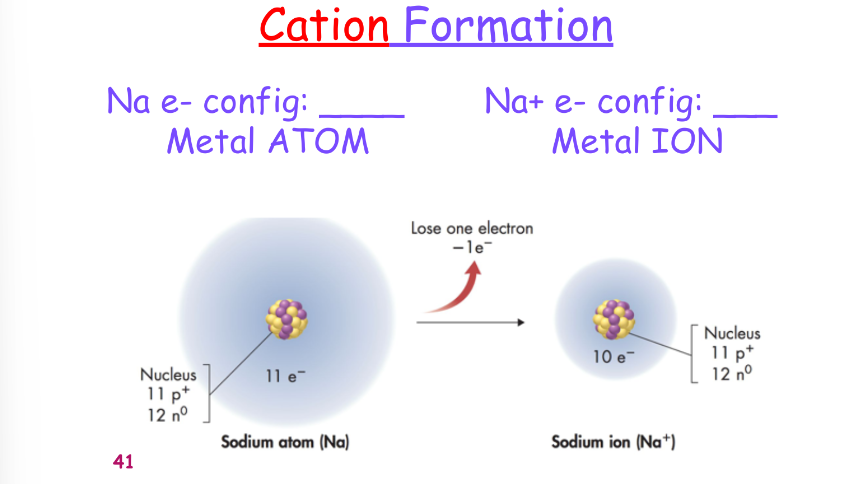
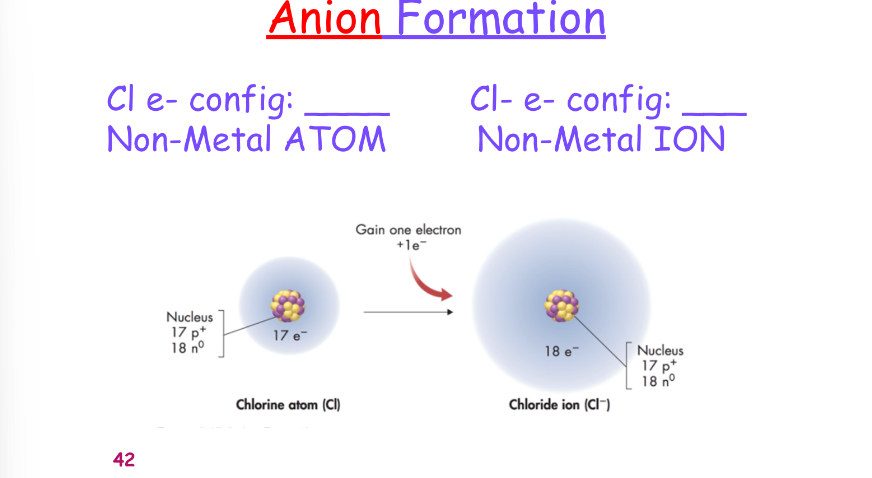
^^cation/anion formation:^^
ionic radius^^: the measure of an ion’s size (it is the measure of distance from an atom’s nucleus to its outermost energy level)^^
- ^^NOT THE SAME AS ATOMIC RADIUS^^
- ^^metal ions^^ generally have ionic radii smaller than atomic radii because they lose electrons from their outer energy shells to form + ions
- ^^nonmetal ions^^ generally have ionic radii larger than atomic radii because they gain electrons in their outer energy shells to form - ions
note: ionic radius is not a consistent property, it varies depending on many factors
UNIT 5 (PART I) ✰
^^chemical bonds:^^
- compounds are a chemical combination of two or more elements in exact ratios (result of a chemical bond, which is a lasting attraction between atoms, formed from a chemical reaction)
- valence electrons are the outermost electrons of an atom- atoms bond according to their electronegativities (achieving stability by having full sets of valence electrons)
^^octet rules^^: a rule of thumb for non-transition metals (end up with 8 valence electrons)
- main group elements (groups 1, 2, 13-18)
- DO NOT USE FOR TRANSITION METALS!
- max. electrons would be 2 per PEL
- atoms would lose, gain, or share electrons order to achieve an octet
- atoms with an octet are more stable and have less energy overall
BARF:- breaking a bond requires absorption of energy- release of energy forms a bond
^^types of bonds:^^
- ionic bonds occur as a result of losing and gaining electrons
- covalent bonds (or molecular bonds) form from the sharing of electrons
- metallic bonds occur uniquely between atoms of metals
^^forming an ionic bond:^^
^^when metals react they:^^
- lose e-
- become positively charged
- have smaller radii
- acquire the e-configuration of a noble gas
^^when nonmetals react they:^^
- gain e-
- become negatively charged
- have larger radii
- acquire the e-configuration of a noble gas
so, ionic bonds typically form from metal + nonmetal because the ions are of opposite charge and are attracted to each other
^^ionic solids:^^
- we call solid compounds formed through ionic bonds ionic solids
properties:
- solid, crystalline structure—organized structure
- high melting points
- electrical conductors when melted or in solution
covalent bonds:
- 2 nonmetals or a metalloid & nonmetal
- sharing of e
- nonpolar (equal sharing)
- polar (unequal sharing)
typically illustrated as a line drawn between atoms
covalent “solids”
self-explanatory: compounds formed with covalent bonds
properties:
- soft
- low melting point
- poor conductors of heat and electricity
special type: network solid
- continuous covalently bonded compound
- extremely hard
- very high melting point
- poor conductors
metallic bonds:
- formed by the attraction of a single metal’s electrons and positively charged nuclei
- one of the reasons why metals are conductive are that they easily lose their electrons
- in a pure metal, the valence electrons can move freely between atoms, creating a “sea of electrons”
- metallic solids = metals
^^polarity:^^
nonpolar covalent: difference of 0.4 or less
polar covalent: difference between 0.4 and 1.8
ionic: difference greater than 1.8
SNAP: symmetric = nonpolar, asymmetric = polar (rotational)
^^intermolecular forces:^^ forces that act between molecules (van der waals forces)
intramolecular forces: forces that act within a single molecule (a.k.a. bonds)
dipole-dipole forces:
- dipole—polar molecules
- “di” = two, + poles
- oppositely charged poles of different atoms will attract
hydrogen bonding: special type of dipole-dipole interaction
- hydrogen is particularly attracted to nitrogen, oxygen, and fluorine (the most electronegative atoms)
- stronger than dipole-dipole interaction
london dispersion forces: (weakest type of intermolecular force)
- caused by random movement of electrons to create temporary dipoles in molecules
- same attraction between poles then repeats the process with the next atom/molecule
- typically occurs in nonpolar molecules and single atoms (noble gases)
- scales with number of electrons/size of molecules and atoms
^^electron dot diagrams:^^ a.k.a. Lewis electron dot diagrams
we only draw valence electrons, and we will follow the octet rule
electrons should only go above, below, or next to the chemical symbol
- electrons 1 and 2 are placed together on any side
- electrons 3, 4, and 5 are spread across the other three sides
- electrons 6, 7, and 8 are paired with the other single electrons
for ions, more information is required:
- indicate charge by putting your element and dots in brackets and writing the charge as a superscript outside
- metal ions should contain no dots in the diagram
- nonmetal ions should show gained electrons as dots
for covalent compounds:
- as we previously have said, draw shared electrons/bonds as lines connecting atoms
- unshared electrons can remain as dots
- make sure to satisfy the octet rule (except for PEL 1)
tips:
- count the total number of valence electrons
- arrange the atoms to show bonds between them
- starting with the atom with the lowest electronegativity in the center (EXCEPT H) and satisfying the octet rule
- distribute the remaining electrons across the atoms that do not have a complete valence shell
- if anything has an incomplete shell, try adding double/triple bonds
^^polyatomic ions:^^
recall: ions are charged particles, this means that you can have more than one atom bonded together that has a charge
polyatomic ions are ions composed of multiple atoms bonded together
- examples can be found in table e
- they can be positively or negatively charged
- each of these can behave like a single atom ion of the same type (+ or -)
UNIT 5 (PART II) ✰
^^chemistry formulas & naming compounds:^^
the basics:
- when we have been showing substances, we have been using chemical formulas, which show qualitative and quantitative information about the substance
- the ^^chemical symbols^^ = elements present
- the ^^subscripts^^ = how many atoms of each element
types of formulas:
^^empirical formula^^ - the simplest ratio in which atoms combine to form a compound
ex.: KCl, CaO, Al2O3, CH2O
^^molecular formula^^ - the formula that tells you what is part of a molecule (the smallest unit of a covalent compound)
ex.: H2O, C6H12O6
this does NOT have to be the simplest ratio of elements
oxidation states/oxidation #:
^^an atom’s oxidation state^^ = its hypothetical charge if you assume all its bonds were fully ionic
ex.: Na has an oxidation state of +1; you can interpret this as Na forms an ion with a 1+ charge in an ionic bond
unless otherwise shown, you should use the top number, which is the most common oxidation state!
naming compounds (pt. 1):
^^binary ionic compounds^^ (2 parts)
- rules for writing formulas: elements with positive oxidation #’s combine with elements with negative oxidation #’s (USE THE TOP # FOR NOW)
- the algebraic sum of all the charges that you have must be zero to reach a compound that is overall neutral
- write the formula starting with the element with the positive oxidation #, then the one with the negative # number
^^“cross & drop”^^
- write the elements and their oxidation states above the respective elements in the appropriate order (positive, then negative)
- take the number of the oxidation state (not the charge!) of one element and write it as the subscript of the other for both elements
- ^^scale^^ the numbers down to the simplest whole number ratio possible
^^giving compounds names^^
for binary ionic compounds, we do the following
- ^^keep^^ the first element (the positive one) as is
- ^^use the suffix “–ide”^^ instead of the normal name of the second element
- ^^ending in -ine, -ium or -on^^: swap the suffix out for “i-de” directly (“carbide,” “chloride,” “telluride”)
- ^^ending in –gen^^: drop the vowel preceding the suffix as well before you swap for “-ide” — ”hydride” “oxide”
weird ones: sulfur = “sulfide,” phosphorus = “phosphide,” antimony = “antimonide,” arsenic = “arsenide”
“what happens if your metals have multiple oxidation states?”
the stock system! - used to indicate specific oxidation states for ^^metals^^ that have multiple oxidation states
- positively charged elements only
- for elements that have ^^multiple oxidation states^^, a roman numeral in parentheses is added to the name to show the one it has in the compound being shown
ex.: FeCl3—iron (III) chloride
remember—all compounds should end up with 0 charge, so use the charge of the other element to help you figure out what you have!
compounds with polyatomic ions:
recall: polyatomic ions are groups of atoms that can ^^behave^^ like single atom ions
ex.:
NaCl - regular ionic compound
NH4Cl - polyatomic ionic compound
NH4NO3 - only polyatomic ions
when ^^multiple of these are involved in forming a compound^^, they need to be shown as part of a single group (we do so by using parentheses to indicate the polyatomic ions where more than one is needed)
ex.: (NH4)2SO4
naming covalent compounds:
FIRST RULE: DO NOT SIMPLIFY CHEMICAL FORMULAS
we will only be dealing with ^^binary molecular compounds^^
prefix naming system - know your prefixes and their associated #’s
1—mono
2—di
3—tri
4—tetra
5—penta
6—hexa
7—hepta
8—octa
9—nona
10—deca
general rules are like naming binary ionic compounds…
- first element keeps its original spelling
- second element is changed to end in –ide
additional rules
- prefixes are required for ^^BOTH^^ elements to show how many of each atom is in the compound
- the exception is using mono- for the first element (not necessary)
weird naming quirk: for oxide, drop any ending “a” or “o
^^kinetic molecular theory:^^
gases basics:
- gases have no definite shape or volume and can be compressed
- gas molecules are constantly moving about and have kinetic energy
- ^^higher temperatures = more kinetic energy^^
- normal boiling point: the temperature at which a substance changes from liquid to gas at atmospheric pressure
bringing intermolecular forces into the mix:
- intermolecular forces are forces of attraction, which means that to get a liquid to become a gas, you must counter these forces
- requires additional energy, which means that ^^the stronger the intermolecular forces in a substance, the higher its boiling point^^
order of strength of IMFs: solid > liquid > gas
kinetic molecular theory (KMT) is a model or theory used to explain the behavior of gases
- describes relationship between pressure; volume; temperature; and velocity, frequency, and force of collisions
main points:
- gases contain particles that are in constant, random, straight-line motion
- gas particles collide with each other and with the container, transferring energy, but not resulting in any loss of energy (perfectly elastic)
- gas particles are very far apart and have negligible volume
- gas particles do not attract each other (no IMFs)
^^gas laws:^^
relationships:
- ^^pressure and how much gas you have^^: more gas particles bouncing off the container = more force = more pressure
- ^^pressure and volume^^: applying more pressure to gases decreases the amount of space they take up; decreasing the amount of space causes more collisions between gas and container
- ^^temperature and pressure^^: temperature is not just proportional to KE, it is defined as a measure of average KE of particles in a substance
(KE = ½ mv^2)
as you increase the temperature, particles hit the container with more energy = more force
- ^^temperature and volume^^: as you increase the temperature, gas particles move faster and will exert more force; if you want to maintain the same pressure, you need to expand the container so that less collisions happen
the combined gas law: (p1v1)/t1 = (p2v2)/t2
p = pressure (measured in torr., mmHg, atm, or kPa)
v = volume
t = temperature (measured in K)
1 - situation 1
2 - situation 2
TEMPERATURE MUST BE IN KELVIN
^^behaviors of ideal vs. real gases:^^
real gases:
- particles have volume
- energy lost in collisions
- intermolecular forces
ideal gases:
- particles have no volume
- collisions are elastic
- no interactions between particles
real gases behave like ideal gases: at high temperatures and low pressures
^^our KMT describes an ideal gas^^
gases are ideal if they behave exactly as predicted.
two assumptions that KMT makes are actually not true of gases:
- KMT: gas particles do not attract one another
reality: most of the time, you can ignore attractive forces because they are small. when conditions become extreme, IMFs matter—^^very cold temperature will cause gases to not behave ideally^^
- KMT: gas particles do not occupy volume
reality: gas particles have very little volume normally, but when you increase the pressure to extreme amounts, the volume matters—^^very high pressures will also cause gases to not behave ideally^^
UNIT 6 ✰
^^nucleus review:^^
- ^^atomic nuclei^^ are made up of positively charged protons and neutral neutrons
- # of protons determines the element
- ^^isotopes^^ are atoms of the same element with different # of neutrons
- nuclear chemistry is chemistry that deals with changes to the nucleus of atoms
- this is the very first type of “^^reaction^^” that we will deal with
^^stability of nuclei:^^
- not all atomic nuclei are stable, meaning that they cannot exist forever when left undisturbed
the full explanation why deals with some extremely complicated physics (^^short answer: stability = minimal amount of energy, instability = not yet at minimum^^)
- an unstable nucleus is ^^radioactive^^—it releases energy by emitting high-speed particles (^^radiation^^)
- any isotope of an element that has an unstable nucleus/is radioactive is known as a ^^radioisotope^^
type 1: very heavy elements—any element above 82 is radioactive
type 2: bad ratio of protons and neutrons
chemical vs. nuclear rxns:
- chemical
- rearrange atoms (^^share & transfer e-^^)
- the nucleus is unchanged
- 2(H2) + O2 → 2(H2)O
- affected by the changes in T, P, or catalysts
- nuclear
- change 1 element into another transmutation
- the nucleus is changed
- not affected by the changes in T, P, or catalyst
REFERENCE: TABLE O
^^natural decay:^^
before/parent nucleus → after/daughter nucleus
natural decay: alpha (α)
speed - slow
mass - 4 amu
charge - +2
^^α-particle = 4/2 He (emission)^^
natural decay: beta (β-)
speed - medium
mass - 0 amu
charge - -1
^^β--particle = 0/-1 e (emission)^^
natural decay: positron (β+)
speed - medium
mass - 0 amu
charge - +1
^^β+ - particle = 0/+1 e (emission)^^
natural decay: gamma (γ)
speed - fast
mass - 0 amu
charge - 0
penetrating powers rank (increasing):
- alpha
- beta
- gamma
- neutron
using table n: shows natural decay processes of several radioisotopes
- radioactive decay is the process by which an ^^unstable atom loses energy^^ via radiation
- natural decay—naturally occurring, not human-induced
important information about natural decay:
- there is always going to be one reactant (starting material)—^^the original radioisotope^^
- there will always be two products—^^the emitted particle and the new isotope^^
- mass and charge must balance out
finding radioactive decay products:
- determine what type of decay the given isotope undergoes
alpha: loses 2n and 2p
beta-: 1n becomes 1p
beta+: 1p becomes 1n
- identify the ^^radioisotope^^ and ^^all necessary atomic information (mass number, atomic number)^^
- apply the change from the proper decay type
- show the change with proper notation
(reactant) → (product 1) + (product 2)
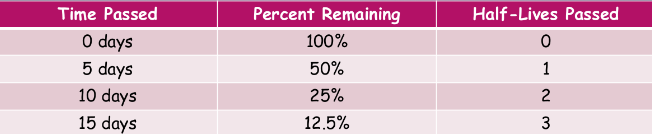
^^half-life:^^
can we calculate when a radioactive atom decays?
we can’t, you can’t influence radioactive decay by changing the conditions surrounding an atom, and it is entirely random
- our best guess is to measure a sample of radioactive atoms to determine how long it takes for half of the atoms in them to decay into the product
- this is the half-life of a radioactive isotope—the amount of time it takes on average for half of the atoms in a sample to radioactively decay
^^transmutations^^%%:%% atoms of one element changing to atoms of a different element
two kinds: natural & artificial
natural - happens spontaneously, radioactive decay, 1 reactant
ex.: uranium-238 → thorium-234 + alpha particle
artificial - requires shooting particles at another particle, 2 reactants
some general physics knowledge:
E = mc^2
E = energy
m = mass
c = the speed of light
(this equation tells us that mass could be converted to energy)
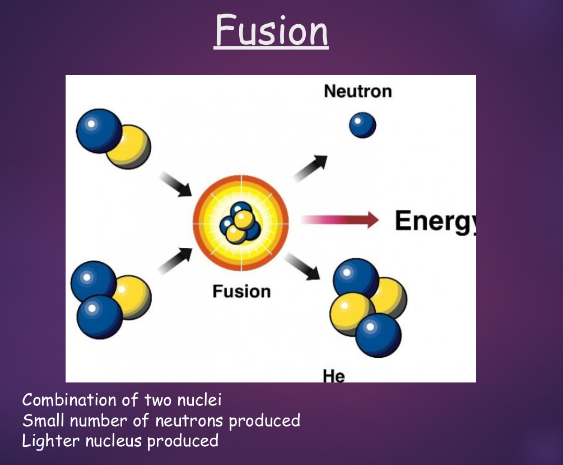
^^nuclear fission/fusion:^^
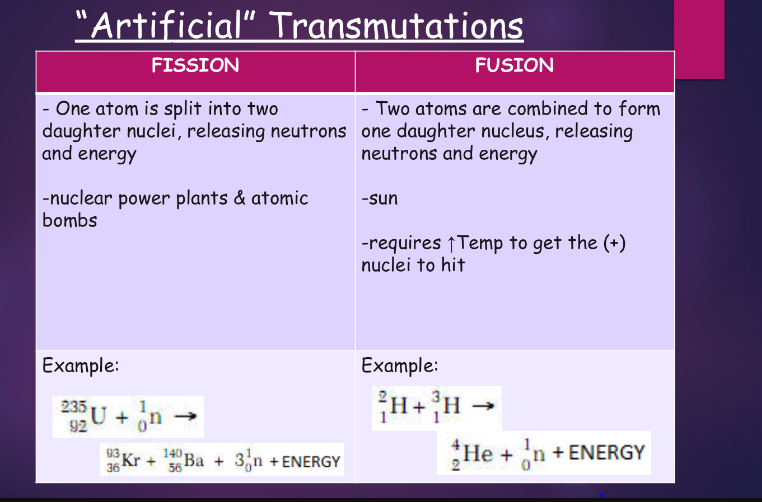
fission:
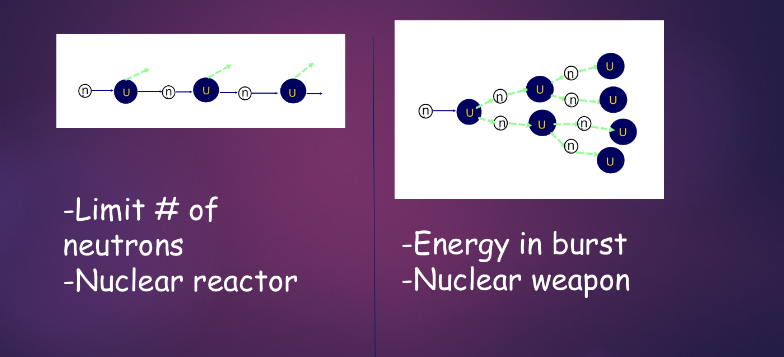
controlled vs. uncontrolled
fusion:
^^applications of nuclear fission and fusion:^^
dating: radioactive dating of things, determining their age
main isotopes
- C-14: used to determine the age of formerly living things up to thousands of years
- U-238: used to determine the age of rocks and minerals
tracers: can used in many fields; medicine, ecology, engineering
- use a radioisotope to see where other atoms of the same element end up
important isotope: P-31
nuclear medicine: mostly to kill cancer and sometimes to take images
important isotopes:
- Co-60 (general cancer killing)
- Tc-99m (general cancer killing)
- I-131 (thyroid specific cancer killing)
nuclear reactors: (self-explanatory)
- very efficient energy source
- less deaths and lower carbon footprint than pretty much any other source of energy
irradiation of food:
important isotope: Cs-137
radiation kills things