Chapter 10: Energy Resources and Consumption
- Humans use both renewable and nonrenewable forms of energy and each has an impact on the environment.
- Humans use energy from a variety of sources, resulting in positive and negative consequences.
Key Terms
- Nonrenewable energy source: Coal, oil, natural gas, and nuclear energy resources that can’t be renewed or regrown for thousands or millions of years.
- Renewable energy source: Wind, biomass, hydrogen, water, and solar energy resources that can be renewed or regrown rather quickly.
- Fossil fuels: Energy sources that come from organisms that lived millions of years ago. After being buried with heat and pressure, they have become coal, oil, or natural gas.
- Peat: Partially decayed plant matter that is used for cooking or heating in parts of Europe.
- Lignite: The least efficient type of coal that generates the least amount of heat and isn’t very common.
- Bituminous: The type of coal used the most because it is very abundant and generates a lot of heat.
- Anthracite: The most efficient type of coal that generates the maximum amount of heat but isn’t very common and is more expensive.
- Cogeneration: Creating electricity and heat from one energy source.
- Turbine: A giant fan-type structure that is turned by steam or water (hydroelectric power) and connected to a generator to make electrical energy.
- Fracking: forcing liquid at very high pressures down into the rock to cause the oil or gas to be released.
- Combustion: The burning of fuel to produce energy.
- Fission: The splitting atoms for energy.
- Uranium 235: The radioactive isotope used to create nuclear power.
- Radioactivity: The radiation emitted from a nuclear isotope.
- Thermal pollution: The heat that is produced by nuclear power generation that can be a pollutant to aquatic organisms.
- Three Mile Island: A nuclear power plant in Pennsylvania where a small nuclear accident occurred, releasing some radiation.
- Chernobyl: A nuclear power plant in the Ukraine where a large nuclear meltdown occurred, releasing a lot of radiation.
- Fukushima: A nuclear power plant in Japan where a tsunami and earthquake caused a release of radiation.
- Half-life: The time it takes for nuclear isotope to lose half of its radioactivity.
- Biomass: Organic matter that can be used for heating or cooking or fuel. Example: wood from trees or ethanol from decaying plant matter.
- Ethanol: A substitute for gasoline that is made from decaying plant matter.
- Photovoltaic solar cell: A cell that captures light from the sun and converts it into electricity.
- Active solar energy: Turning solar energy into electricity or heat and can be stored.
- Passive solar energy: Using the sun to heat or blocking the sun to cool a house or building.
- Hydroelectric power: Using the power of a river or tides to turn a turbine to generate electricity.
- Geothermal energy: Using the heat below the surface of the Earth to make steam to turn a turbine to generate electricity.
- Hydrogen fuel cells: Taking H2 and O2 and producing water, heat, and electricity.
- Kinetic energy: The energy of motion as in the turning of a turbine.
Renewable and Nonrenewable Resources
- Renewable energy resources are resources that can be replaced over a relatively short time scale. These include things like solar, wind, hydroelectric, geothermal, and biomass resources.
- Nonrenewable energy resources are not replaced quickly or not at all. These include things like coal, oil, natural gas, and nuclear energy resources.
Global Energy Consumption
- Developed countries use a lot more energy resources than developing countries, but as a country goes through the demographic transition and becomes more industrialized, they will begin to demand more and more energy.
- Currently, the major source of energy in the world comes from fossil fuels such as coal, oil, and natural gas.
- However, this depends on regional availability of other sources like geothermal, solar, and water or any government regulations that might influence the type of energy source a population uses or has access to.
Fuel Types and Uses
We use many different types of fuel. This can be a family burning wood or charcoal to cook or heat their houses or using peat, partially decayed plant matter that is burned for cooking or heating in parts of Europe. Of course, fuel can also be coal, oil, and natural gas.
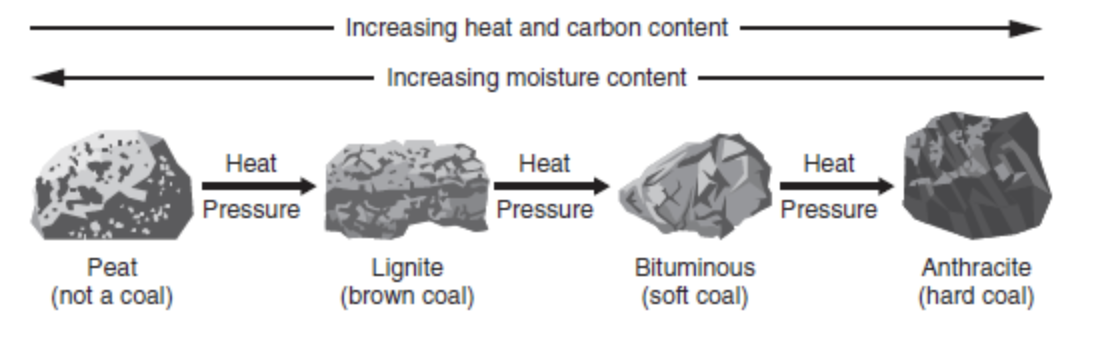
There are different types of coal such as lignite, bituminous, and anthracite (Figure 10.1). As we move from lignite to anthracite, the coal gets harder and burns hotter.
- Anthracite is the highest rank of coal. It has the highest amount of carbon and the lowest amount of volatile matter.
- Lignite is the lowest rank of coal with the least amount of carbon.
- Bituminous coal is the most abundant and extensively used.
Oil or crude oil is made up of hydrogen and carbon with some other trace elements.
- It was formed millions of years ago as dead marine organisms sunk to the bottom of the ocean and under heat and pressure were changed to oil.
- We drill down to the oil reserves and pump it out. We can also find crude oil in tar sands, where a mixture of sand, clay, and water is saturated with petroleum known as bitumen.
- Oil is then burned for energy.
Natural gas is the fossil fuel that burns the cleanest and can be used for energy.
- It is made up mostly of methane but contains other things like carbon dioxide, nitrogen, and more.
- Natural gas can be used to create electricity, heat buildings, and fuel cars.
When an energy source is used to generate both heat and electricity, we say it is a combined heat and power (CHP) system or cogeneration.
Distribution of Natural Energy Resources
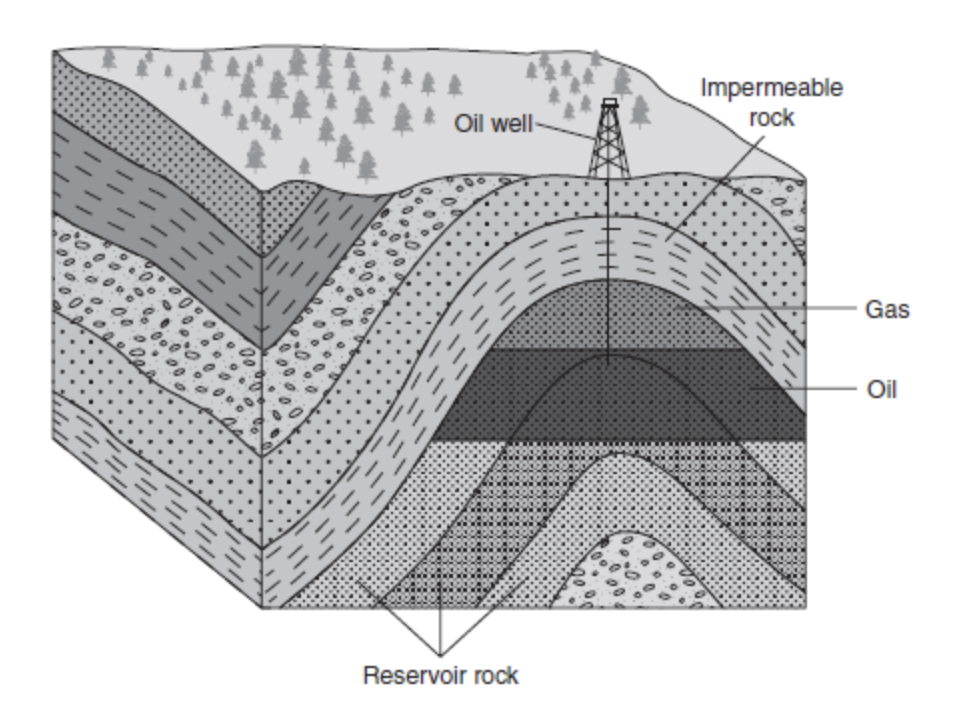
If we look at Figure 10.2, we can see where oil and gas energy sources are found.
- First, the oil is found in a layer below the gas. This is because gas bubbles up from the oil and floats on top.
- The rock reservoir is the layer that has the rich organic matter from millions of years ago. This has then been subjected to heat and pressure.
- The oil has risen up through the next permeable layer but stopped at the layer that is impermeable.
- We then drill down through the impermeable layer and get the gas and oil.
Fossil Fuels
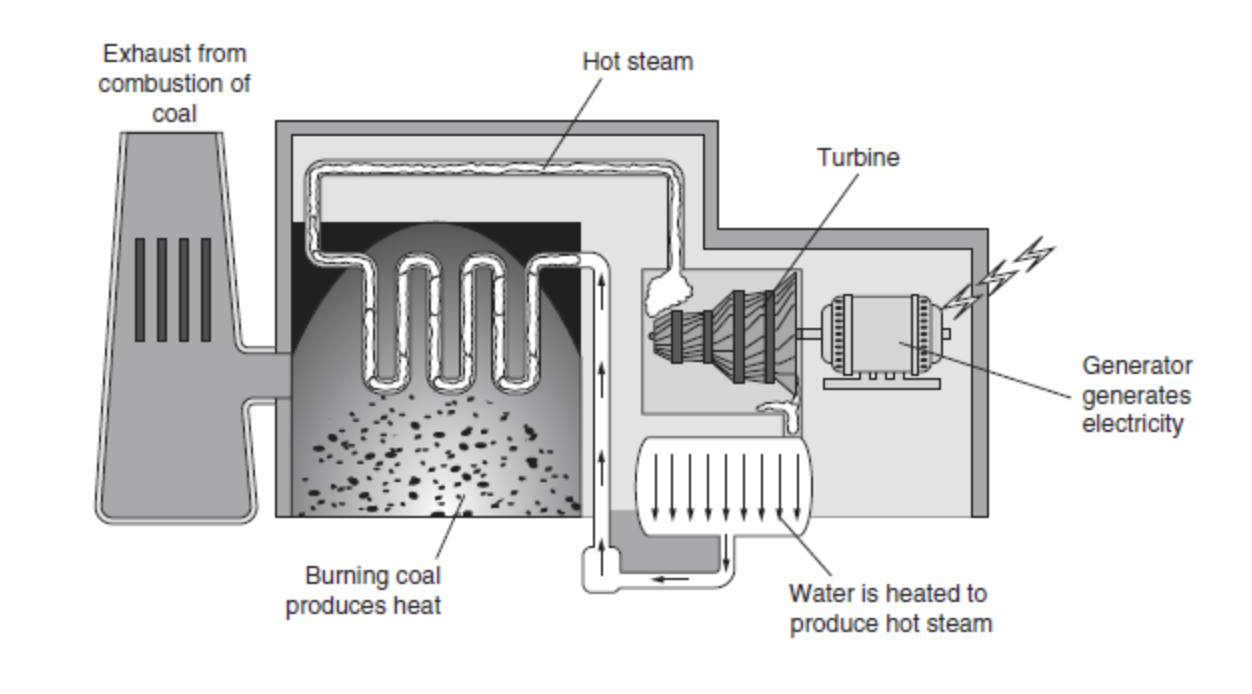
We get energy for electricity from a chunk of coal, or from a gallon of oil or natural gas because these energy items have stored energy, we can turn that stored energy into electrical energy.
- Combustion of these fossil fuels is burning the fossil fuel, which is a chemical reaction between fuel and oxygen, and turning it into carbon dioxide, water, and energy.
- Take coal as an example. First coal is pulverized into a very fine powder, which is then burned to make heat.
- This heat is used to boil water and produce steam.
- This steam is then pumped through a pipe at extremely high pressures and is focused on a giant turbine, causing the turbine to spin (similar to blowing on a pinwheel and causing it to spin).
- This turbine is connected to an electrical generator that generates electricity.
- The same is done when oil or natural gas is burned.
- Figure 10.3 shows a typical coal energy generator.
When we burn fossil fuels we produce carbon dioxide and water. This carbon dioxide is a contributor to climate change that we will discuss in future chapters.
Another environmental problem of the use of fossil fuels for energy is caused when a method called hydrologic fracturing, commonly known as fracking, is used for extracting it.
- Fracking forces liquid at very high pressures down into the rock, which causes the oil or gas to be released.
- This can lead to groundwater contamination because the water that is used to release the oil or natural gas can contain contaminates that then run off into waterways.
- In addition, this process releases volatile organic compounds during the natural gas production process.
Nuclear Power
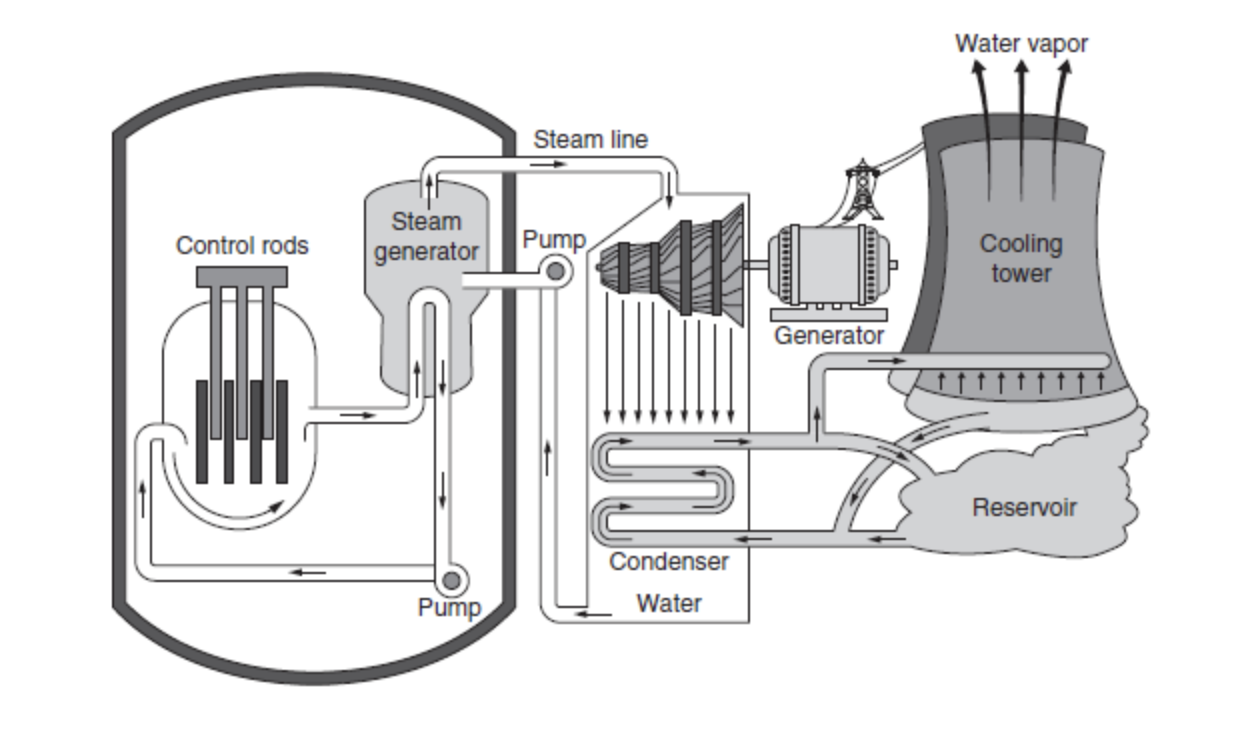
We can also split atoms of Uranium-235, in a process known as fission, to produce extremely high heat.
Similar to how electricity is generated from coal, oil, or natural gas, the heat is used to boil water to make steam to turn a turbine to generate electrical energy. Figure 10.4 is a picture of a typical nuclear power plant.
The uranium-235 is stored in fuel rods and the uranium atoms produce radioactivity as the isotopes lose energy.
- There are several cons of nuclear energy. First, when we use uranium, the waste that is left over must be stored for a very long time. This waste is radioactive and must be very carefully disposed of.
- In addition, uranium-235 is a nonrenewable resource that must be mined; we discussed the environmental problems with mining in the last chapter.
- Also, nuclear energy generation creates so much heat that it must be “dumped” into a water source, which causes thermal pollution that we will discuss in a future chapter.
- However, the pro of nuclear energy is that because we aren’t actually burning anything, no air pollution is produced and therefore this energy source does not cause climate change.
There have been three times in our history when a nuclear accident happened and radiation was released.
- The first was at Three-Mile Island in Pennsylvania, the second in Chernobyl in Ukraine, and the final one at Fukushima, Japan.
- In each of these cases radiation was released from the fuel rods and the environment was impacted, sometimes devastatingly.
Half-life is a term used to describe the amount of time it takes for a radioactive isotope to be half of its original value.
- We use this to calculate how long we must store the radioactive waste, and how radioactive the object is and for how long. For example, uranium-235 has a half-life of 700 million years!
- That’s a long time to store radioactive waste and a lot of time for this waste to harm the environment.
Energy from Biomass
- People in developing nations may not have access to good, reliable energy to cook and heat their homes.
- In this case, many will burn different types of biomass because it is cheap and usually easy to get.
- This might be as simple as burning wood from trees.
- However, this practice while inexpensive and abundant can cause some human health and environmental problems.
- For example, openly burning wood in a closed home or hut produces carbon dioxide, carbon monoxide, nitrogen oxides, particulates, and volatile organic compounds.
- None of these is good to be breathing for long periods of time and many lead to climate change. In addition, if a country is doing this on a large scale there are problems of deforestation and habitat destruction.
- Another type of biomass fuel comes from ethanol. Ethanol is made by fermenting things like corn, wheat, potatoes, and so on.
- Because it can be grown over and over, it is a renewable resource and is used as an alternative to gasoline.
- It has a 34 percent average reduction in greenhouse gases that lead to climate change over gasoline.
- However, ethanol doesn’t have a very large return of energy on investment and if we are converting natural areas of forest and grassland into large farms to produce corn to make ethanol, we are contributing to a problem of habitat loss and greater pollution due to agriculture.
Solar Energy
- The sun is a huge, endless provider of free energy. We can capture this energy to create electricity or we can passively use this energy to heat our homes.
- Photovoltaic solar cells capture and convert solar energy from the sun into electrical energy. The limitations of these cells are that they can only generate energy when the sun is out.
- However, we can use active solar systems to collect, store, and convert solar energy to electrical power. They do this by using heat from the sun to heat a liquid and store the energy.
- The pros of this system are that the energy is free and unlimited and that air pollution isn’t generated because you aren’t burning anything.
- However, these systems are often cost-prohibitive and if you build large solar “farms” with hundreds or thousands of large solar panels in a sunny place like a desert, you could be harming that fragile ecosystem.
- Passive solar systems do not collect or store energy but can be used to heat or cool homes and other structures.
- An example is building a home with large windows that face where the sun shines the most.
- Then, the sun will come in through the windows and heat the home.
- During the summer the homeowner could put thick curtains on the windows to prevent the heat from coming in as well.
- Another option is planting shade trees to help keep a house cooler in summer.
Hydroelectric Power
- Another form of renewable energy is using the power of running water to create electricity. This can be done by building a dam across a river; the dam has a turbine that the water passes over as it goes downstream.
- This turbine turns and is connected to a generator that converts energy of motion into electrical energy. Another way to generate electricity is to use the movement of the tides to turn a turbine.
- The ocean tides come in and out every day; in some places this large movement of water is greater than in others.
- If the movement is large enough a turbine can be built and again it can be connected to a generator to generate electricity.
- The pro of hydroelectric power is that it doesn’t produce any air pollution since nothing is burned. The cons are that building dams is expensive, and when you build a dam you change the river ecosystem.
- You flood an area above the dam, which was once a land habitat. If some of the water is diverted to irrigation, you reduce the flow of water downstream, reducing the amount of nutrients available and changing the ecosystems.
- In some cases the flow is so reduced that the water never reaches the sea. This can damage habitats and cause loss of biodiversity.
Geothermal Energy
- Our planet has a huge amount of heat down in the subsurface of the Earth. If we can access this heat we can create steam and, again, turn a turbine to create electrical energy with a generator.
- We can also use geothermal heat pumps to take that heat to the surface and heat buildings with it. The pros of geothermal energy are it is renewable and very efficient.
- The cons are it is expensive, can release hydrogen sulfide, requires a lot of water, and isn’t readily available everywhere in the world.
Hydrogen Fuel Cell
- A hydrogen fuel cell is a device that converts potential energy into electrical energy.
- Fuel cells can be used in transportation and power.
- The cell uses hydrogen gas (H2) and oxygen gas (O2) and the products are water, heat, and electricity.
- Hydrogen is oxidized at the anode and flows to the cathode where oxygen reacts with hydrogen to make water.
- The advantage of hydrogen fuel cells is they produce no carbon dioxide as they make electricity.
- The problems are they are still very expensive and, in order to create the hydrogen gas, a process called electrolysis is used, which requires energy possibly produced from nonrenewable sources such as coal.
Wind Energy
- Another way to generate free, renewable energy is to capture the energy from the wind to turn a turbine.
- Kinetic energy is energy of motion; if we allow the wind to turn a turbine and turn it into electrical energy, we can use that kinetic energy.
- This method is especially good off the coasts of continents where the wind blows stronger and more often than on land.
- The pros of this type of power is it is clean, is free, and can be used in areas where you are doing other things such as ranching or growing crops.
- A con is that migrating birds, butterflies, and bats have been known to get caught in the turbines and killed.
- This problem can be mitigated by slowing the turbines down or shutting them off during migration times but then an alternate energy source will be needed at those times.