AP CHEM Solubility Rules and Exceptions + Polyatomic ion
1/4
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
5 Terms
All soluable in water
Group 1 ions
Li, Na, K, Rb, Cs, Fr
All have a plus one charge
Ammonium( NH4 -)
Nitrate( NO3 -)
Acetate( C2H3O2 -) OR ( CH3COO-)
Chlorate( ClO3-)
Ions with exceptions (of those that form soluble compounds)
Halides:
Cl-
Br-
I-
Exceptions: Insoluble when
when combined with Ag+ (silver)
Pb 2+ (lead)
Hg2 2+ (mercury)
Sulfates:
SO4 2-
Exceptions: insoluble when
when combined with Ag+ (silver)
Ca 2+ (calcium)
Sr 2+ (strontium)
Ba 2+ (barium)
Pb 2+ (lead)
Ions that are Insoluble in water and their exceptions
Carbonate ( CO3 2-)
→ when combined with group 1 ions or ammonium
Chromate (CrO4 2-)
→ When combined with group 1 ions, ammonium (NH4), Ca 2+, or Mg 2+
Phosphate (PO4 3-)
→ When combined with group 1 ions or ammonium (NH4)
Sulfide( S 2-)
→ When combined with Group 1 ions or ammonium(NH4)
Hydroxide (OH-)
→ When combined with group 1 ions, ammonium (NH4), Ca 2+, Ba 2+, and Sr 2+
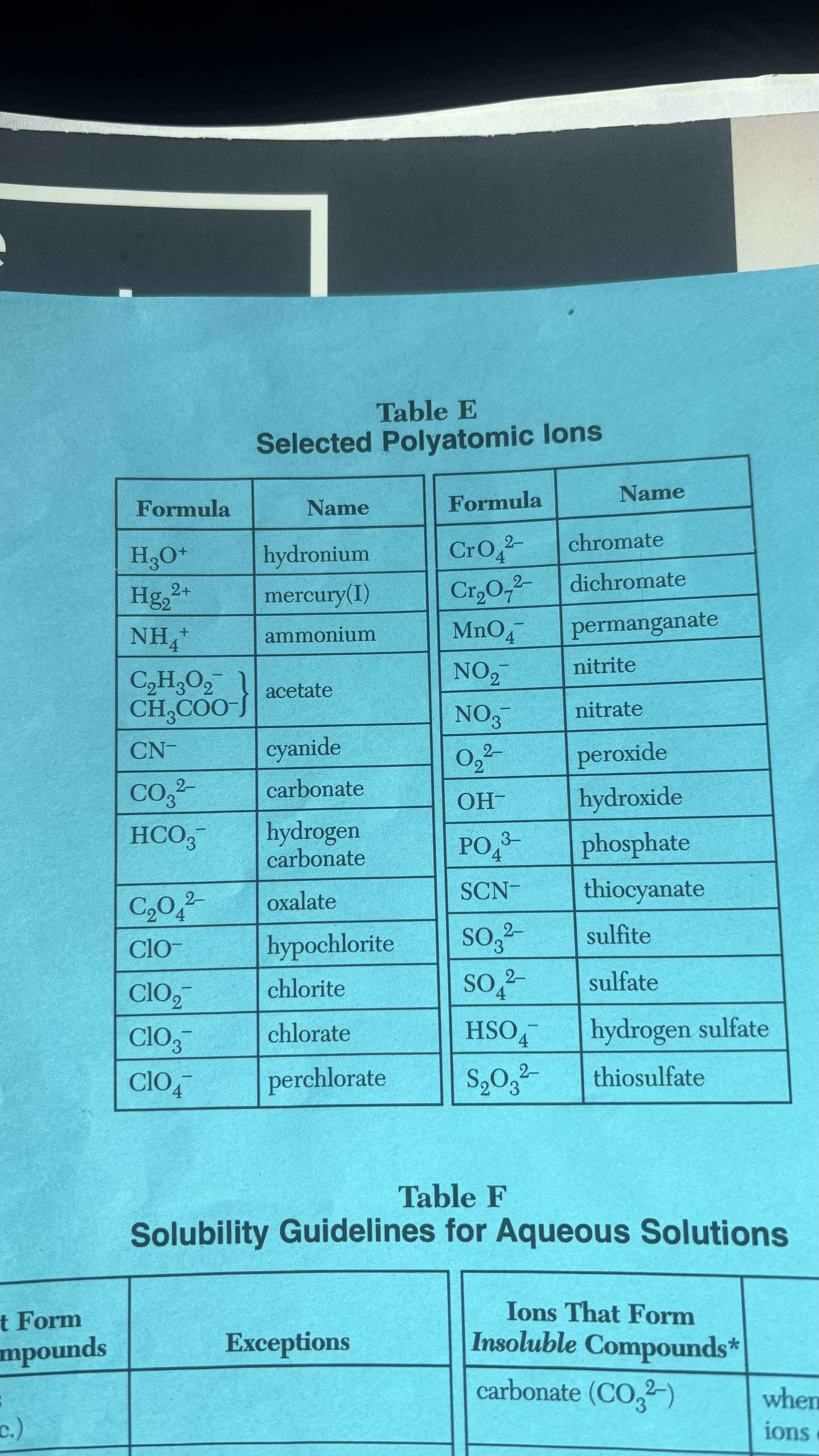
Polyatomic ions
Study as well