AP Chem Test 4A
1/31
Earn XP
Description and Tags
Topics 4.1 to 4.5
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
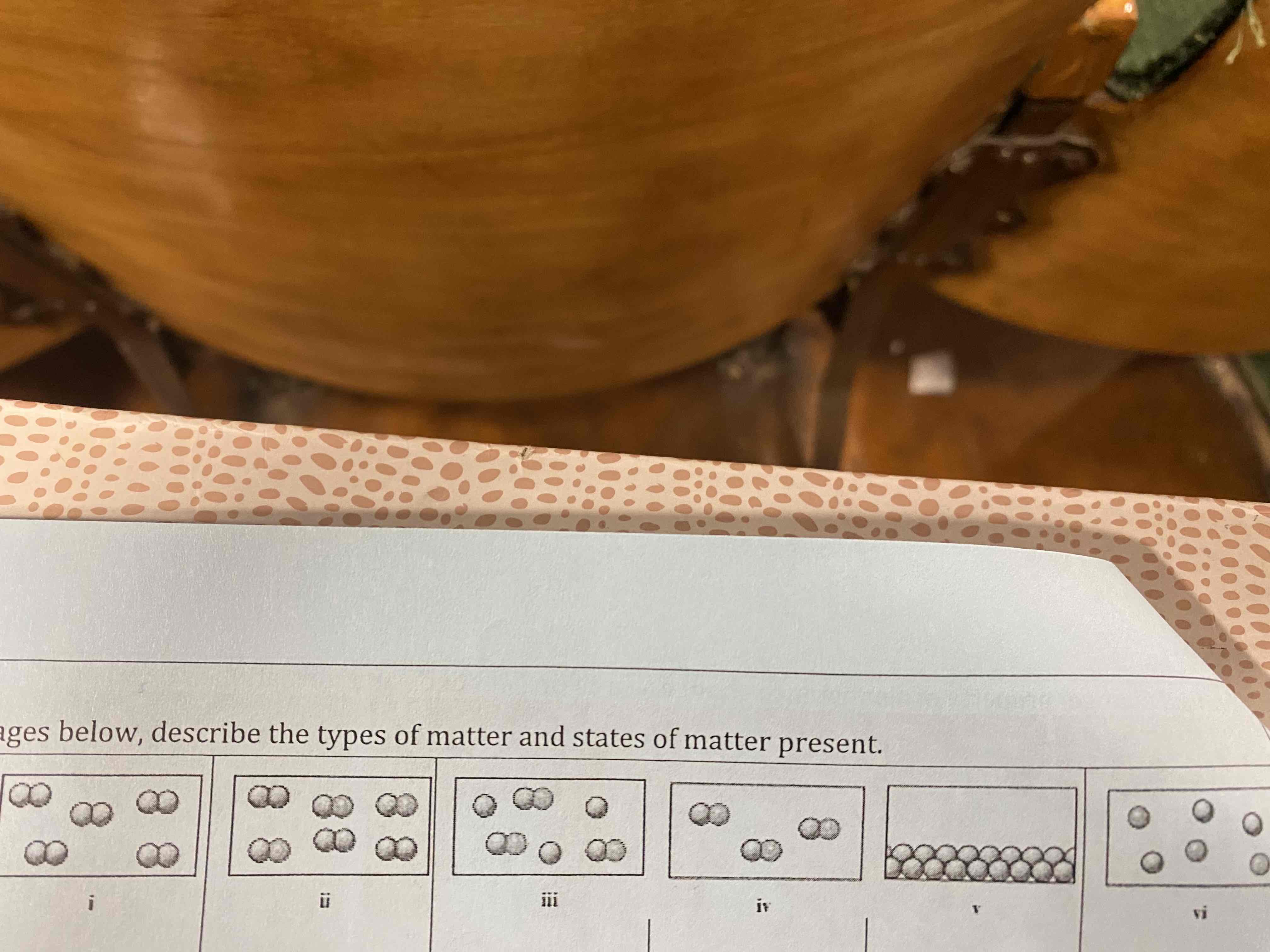
Using the images below, describe the types of matter and states of matter present.
i) element, gas
ii) mixture of an element and a compound, gases
iii) mixture of an element and a compound, gases
iv) compound, gases
v) element, solid
vi) mixture of elements, gases
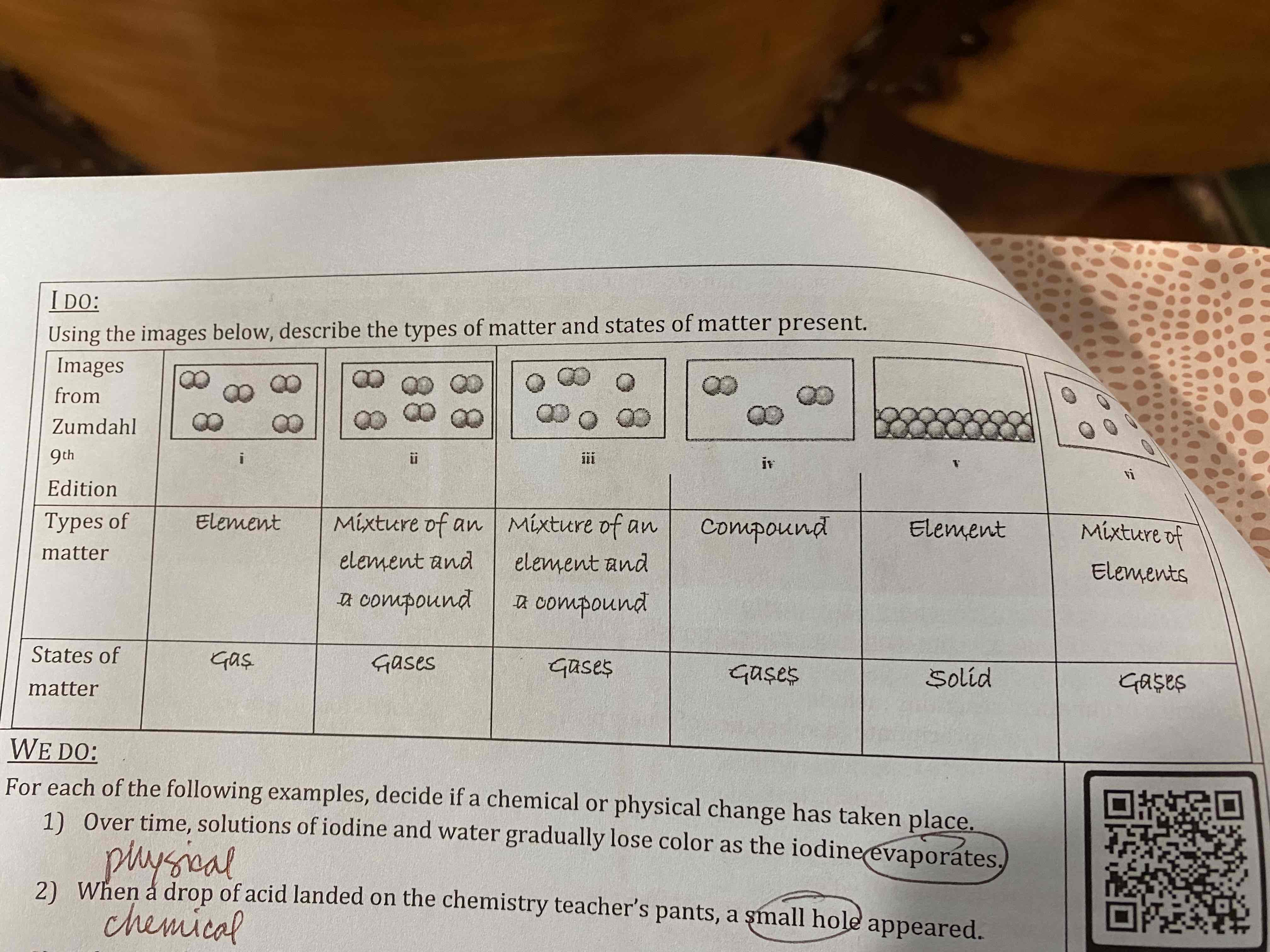
1) Over time, solutions of iodine and water gradually lose color as the iodine evaporates.
2) When a drop of acid landed on the chemistry teacher’s pants, a small hole appeared.
3) When a solution of lead (II) nitrate and a solution of sodium chloride are mixed, a cloudy white precipitate forms.
4) At Halloween, many people use Dry Ice, solid carbon dioxide, in their decorations. It sublimes into a gas and creates a foggy effect.
1) physical
2) chemical
3) chemical
4) physical
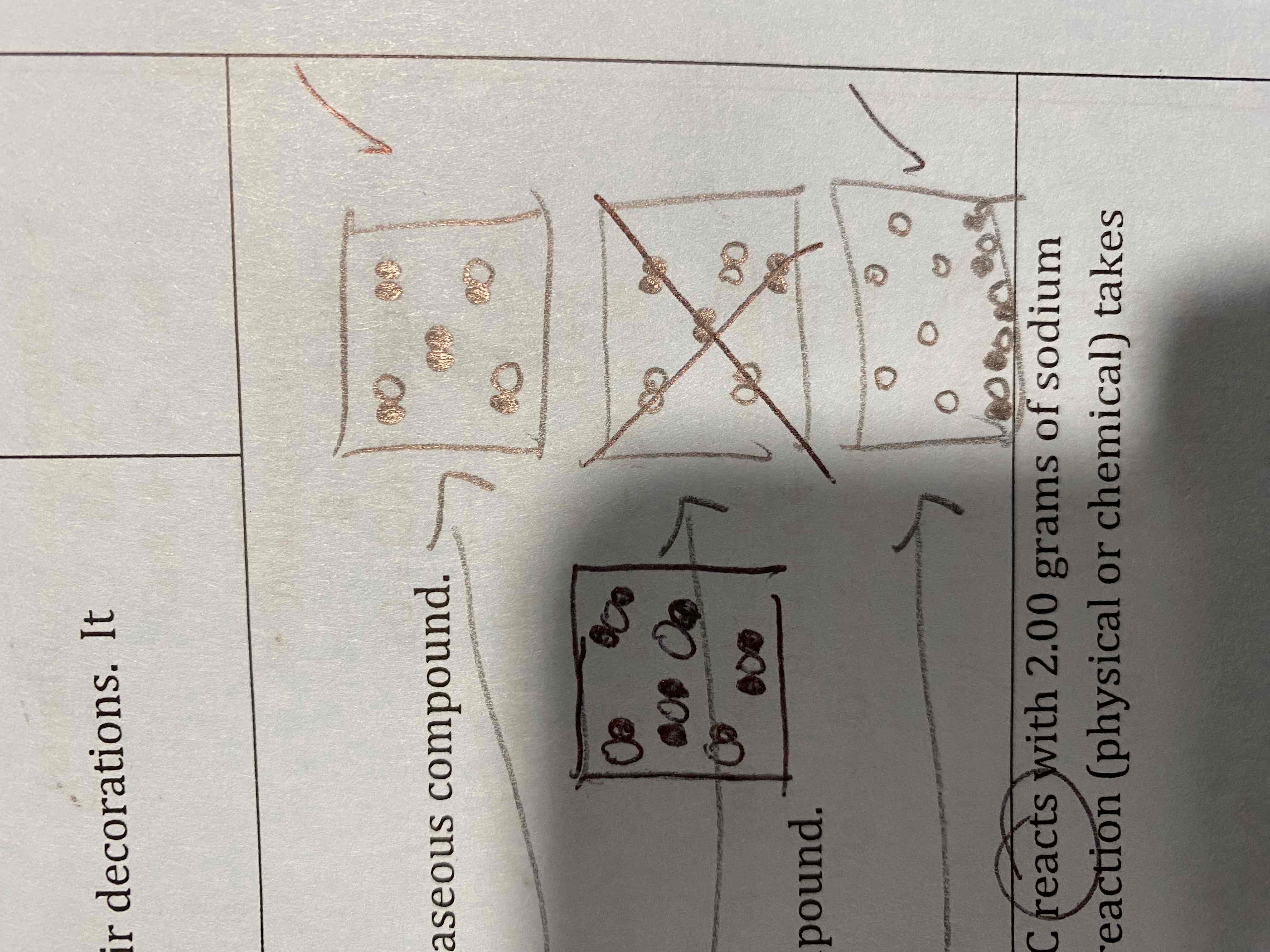
Make a molecular level (microscopic) drawing for the following:
a) A homogeneous mixture of a gaseous diatomic element and a gaseous compound.
b) A homogenous mixture of two gaseous compounds.
c) A heterogenous mixture of a gaseous element and a solid compound.
When 100.0 mL of a 0.50 M solution of hydrochloric acid, HCl, at 22.0 C reacts with 2.00 grams of sodium hydroxide, NaOH, the temperature increases by 12.5 C. What type of reaction (physical or chemical) takes place? How do you know?
Chemical reaction; change in chemical composition due to temperature rise
Identify each of the following as chemical or physical change:
a) 2H2O(g) —> 2H2(g) + O2(g)
b) NH4Cl(s) —> NH3(g) + HCl(g)
c) H2O(s) —> H2O(l)
d) C8H8(l) —>2C4H4(g)
e) 2Al2O3(s) —> 4Al(s) + 3O2(g)
f) C3H6O(l) —> C3H6O(g)
g) NaHCO3(s) + HCH3COO(aq) —> CO2(g) + H2O(l) + NaCH3COO(aq)
a) chemical
b) chemical
c) physical
d) chemical
e) chemical
f) physical
g) chemical
For each of the following descriptions, decide if the material is likely to be a mixture or a pure compound or element, and then decide if the process is a chemical or a physical change.
a) A clear, colorless, crystalline solid is heated. A pale yellow-green has us given off and a shiny, malleable metal was left behind.
b) A brown liquid is heated and a colorless, aromatic liquid is produced along with a clear, colorless liquid while a brown liquid is left behind in the container.
c) Adding sugar to tea cause the tea to taste sweeter.
d) A shiny, dark gray metal and a clear colorless gas combine to form a flaky orange solid.
a) compound, chemical change
b) mixture, physical change
c) mixture, physical change
d) compound, chemical change
A solution of ammonium carbonate, (NH4)2CO3, reacts with a solution of silver nitrate, AgNO3, to form a white silver carbonate precipitate as shown in the image.
Write the balanced chemical equation:
Write the overall ionic equation:
Identify the spectator ions:
Write the net ionic equation:
(NH4)2CO3(aq) + 2AgNO3(aq) —> Ag2CO3(s) + 2NH4NO3(aq)
2NH4+(aq) + CO3-2(aq) + 2Ag+(aq) + 2NO3-(aq) —> Ag2CO3(s) + 2NH4+(aq) + 2NO3-(aq)
2NH4+(aq) + 2NO3-(aq)
CO3-2(aq) + 2Ag+(aq) —> Ag2CO3(s)
Copper (II) nitrate, Cu(NO3)2, solution reacts with potassium hydroxide, KOH, to form a blue precipitate of copper (II) hydroxide.
Write the balanced chemical equation:
Write the overall ionic equation:
Identify the spectator ions:
Write the net ionic equation:
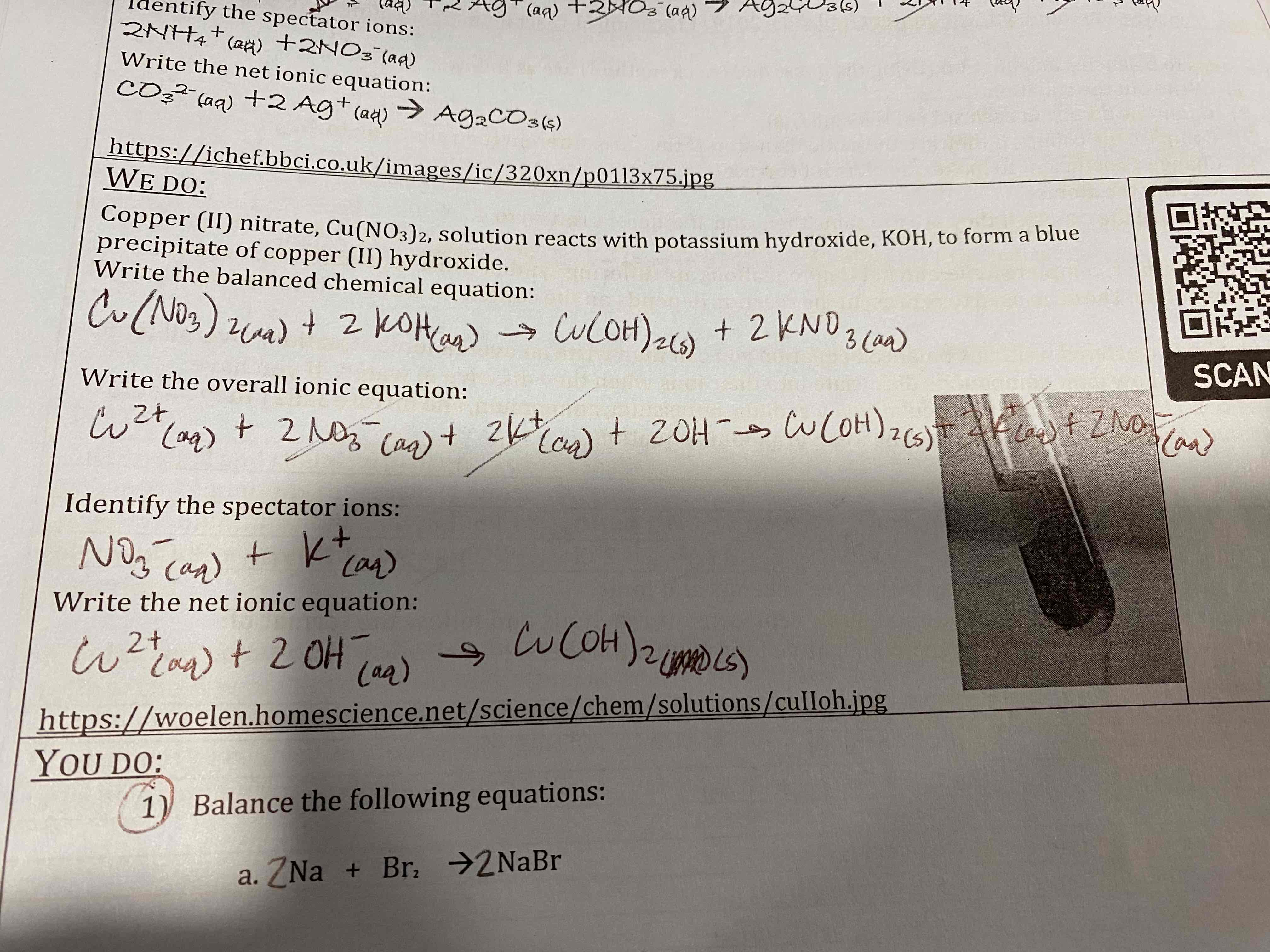
Balance the following equations:
a. Na + Br2 —> NaBr
b. HClO3 —> ClO2 + H2O + O2
c. C4H10 + O2 —> CO2 + H2O
d. Sb2S3 + HCl —> SbCl3 + H2S
e. Al + HCl —> AlCl3 + H2

Write the net ionic equations for the reaction that occurs when aqueous solutions of ammonium sulfate, (NH4)2SO4, and barium nitrate, Ba(NO3)2, react to form a white precipitate.
a. Write the balanced chemical equation:
b. Write the overall ionic equation:
c. Identify the spectator ions:
d. Write the net ionic equation:

Write net ionic equations for the reaction that occurs when aqueous solutions of lithium hydroxide, LiOH, with hydrobromic acid, HBr, undergo a neutralization reaction that produces salt and water.
a. Write a balanced chemical equation:
b. Write the overall ionic equation:
c. Identify the spectator ions:
d. Write the net ionic equation

Write net ionic equations for the reaction that occurs when aqueous solutions of potassium nitrate, KNO3, and potassium hydroxide, KOH, are combined. A clear solution results.
a. Write the balanced chemical equation:
b. Write the overall ionic equation:
c. Identify the spectator ions:
d. Write the net ionic equation

In some rare cases, two different precipitates can form in the same reaction. *insert reaction*
Write the overall and net ionic equation (it is the same reaction) for the reaction above.

When zinc metal, Zn, is added to hydrochloride acid, HCl, the rapid production of hydrogen gas, H2, occurs.
a. Write the balanced chemical equation:
b. Write the e overall ionic equation:
c. Identify the spectator ions:
d. Write the net ionic equation:

Dry ice is solid carbon dioxide, CO2, which will sublimate at room temperature. Write the balanced chemical equation that represents the phase change of dry ice.
CO2(s) —> CO2(g)
Water is seen here on earth in all three states of matter. Write the balance chemical equation that shows:
a. Liquid water freezing
b. Boiling
c. Ice melting
d. Snowflakes forming from water vapor in the clouds

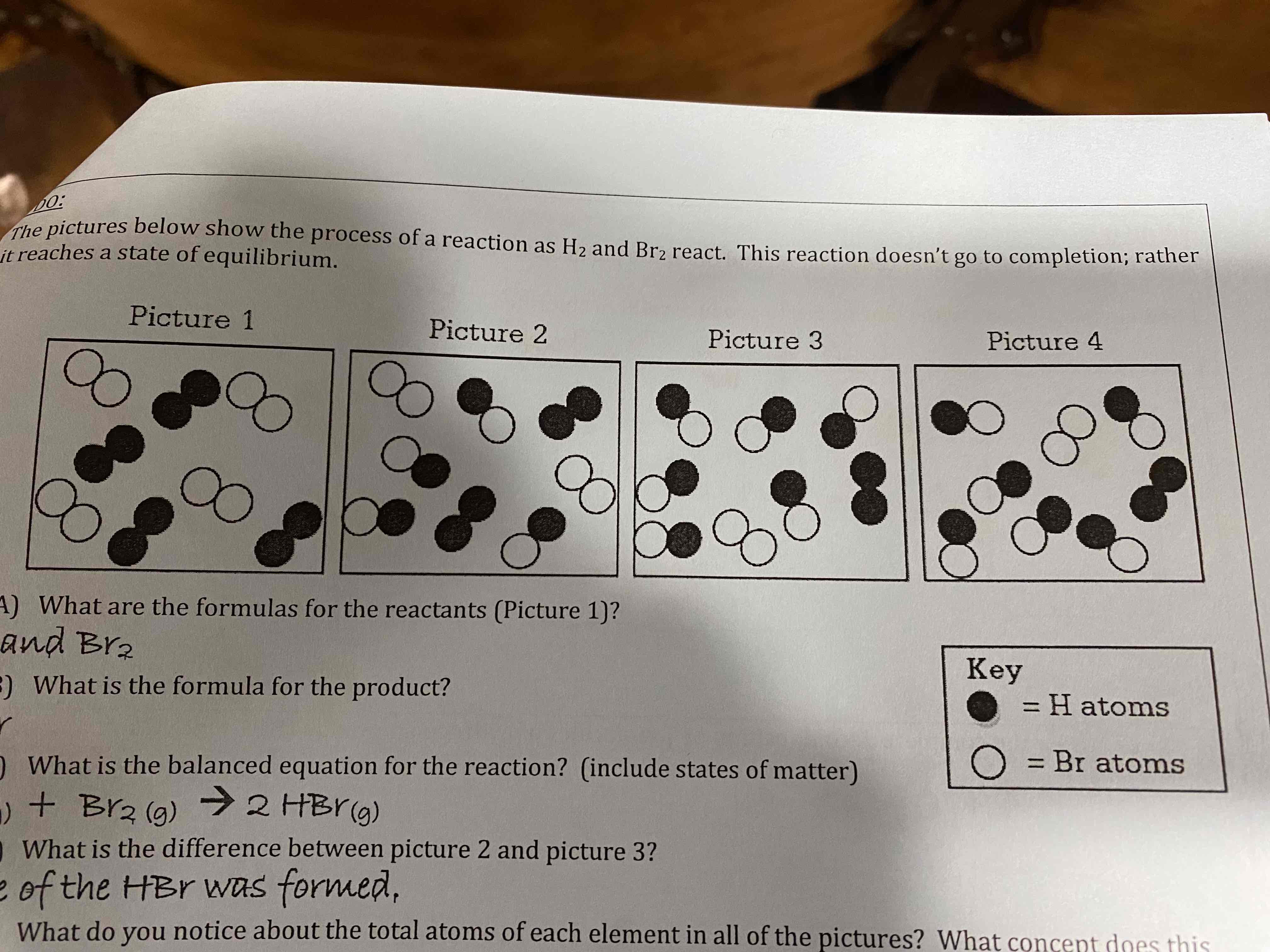
The pictures below show the process of a reaction as H2 and Br2 react. This reaction doesn’t go to completion; rather it reaches a state of equilibrium.
A) What are the formulas for the reactants (Picture 1)?
B) What is the formula for the product?
C) What is the balanced equation for the reaction? (include states of matter)
D) What is the difference between picture 2 and picture 3?
E) What do you notice about the total atoms of each element in all of the pictures? What concept does this represent?
A) H2 and Br2
B) HBr
C) H2(g) + Br2(g) —> 2HBr(g)
D) More of the HBr was formed.
E) The total number of each type of atom is consistent throughout; thus demonstrates the law of conservation of matter/mass.
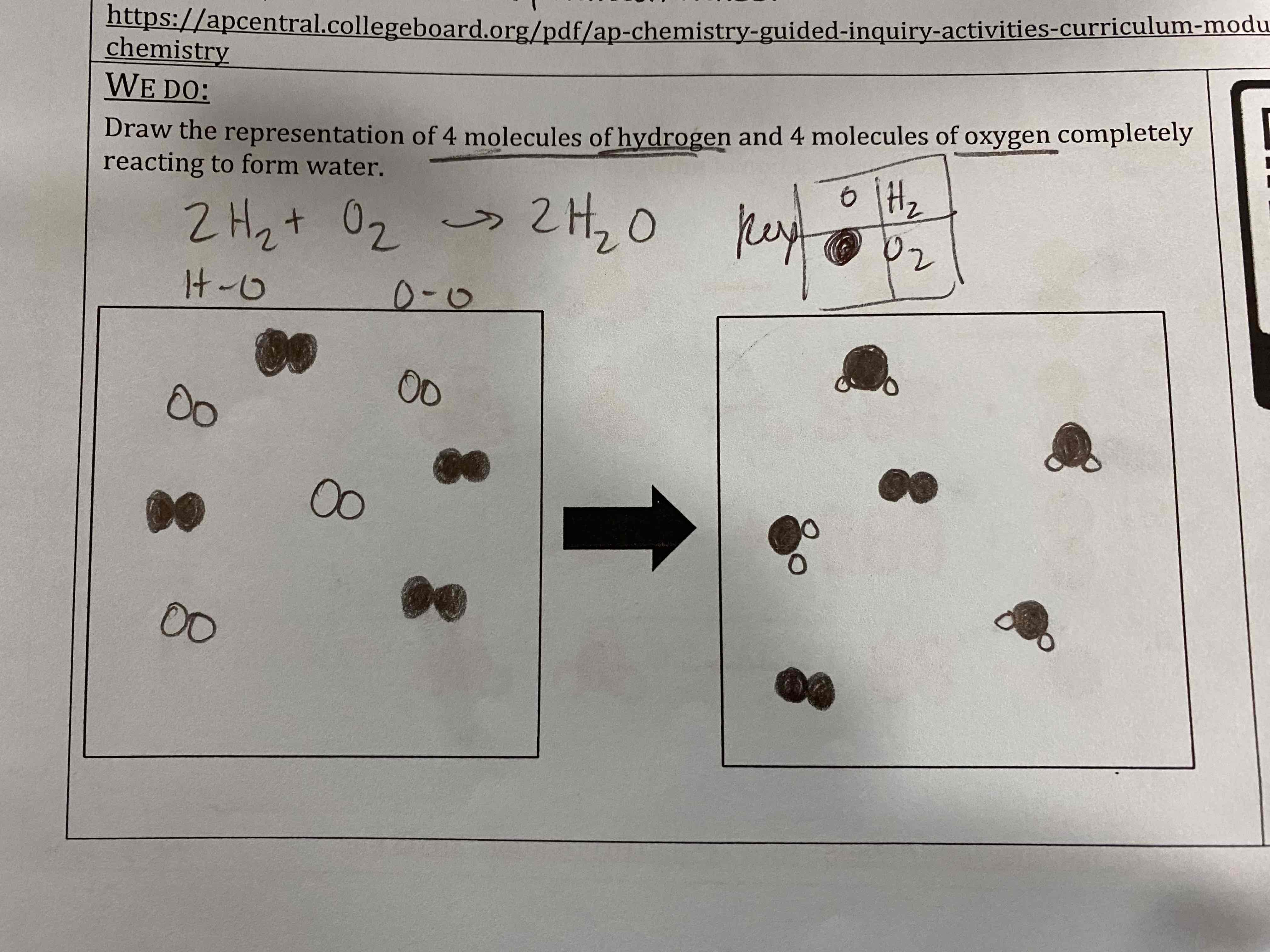
Draw the representation of 4 molecules of hydrogen and 4 molecules of oxygen completely reacting to form water.
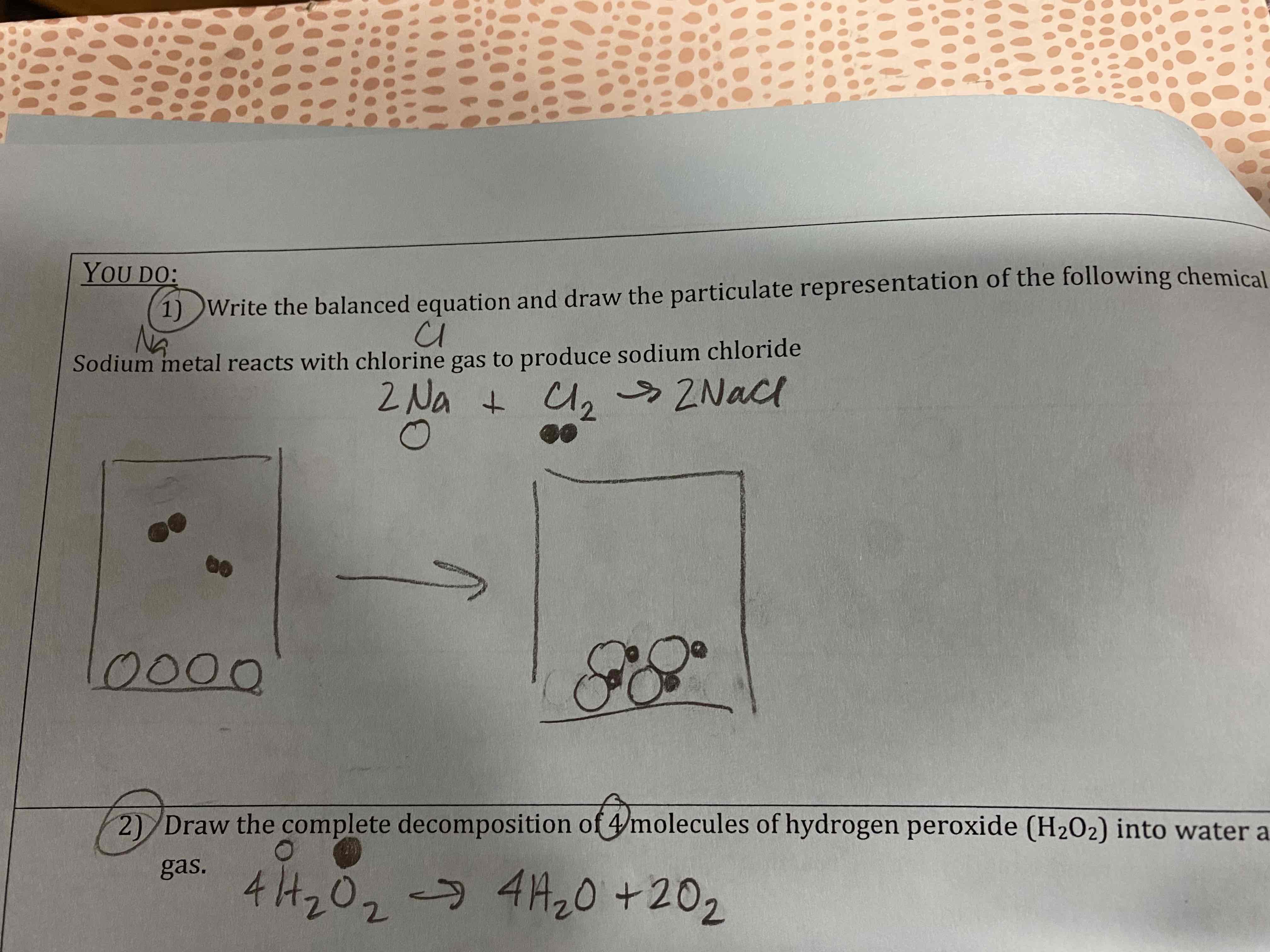
1) Write the balanced equation and draw the particulate representation of the following chemical reaction: Sodium metal reacts with chlorine gas to produce sodium chloride.
Draw the complete decomposition of 4 molecules of hydrogen peroxide (H2O2) into water and oxygen gas.
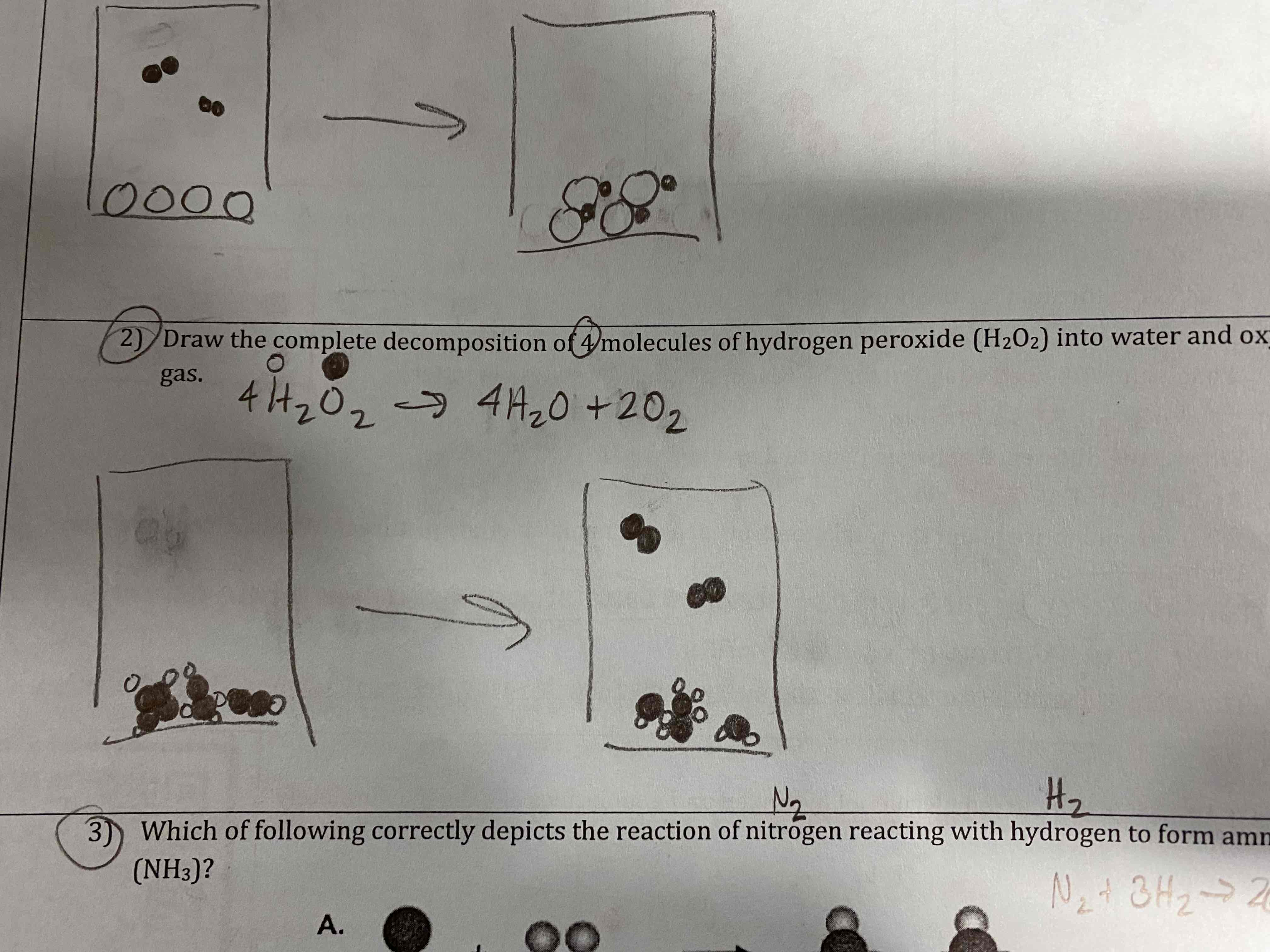
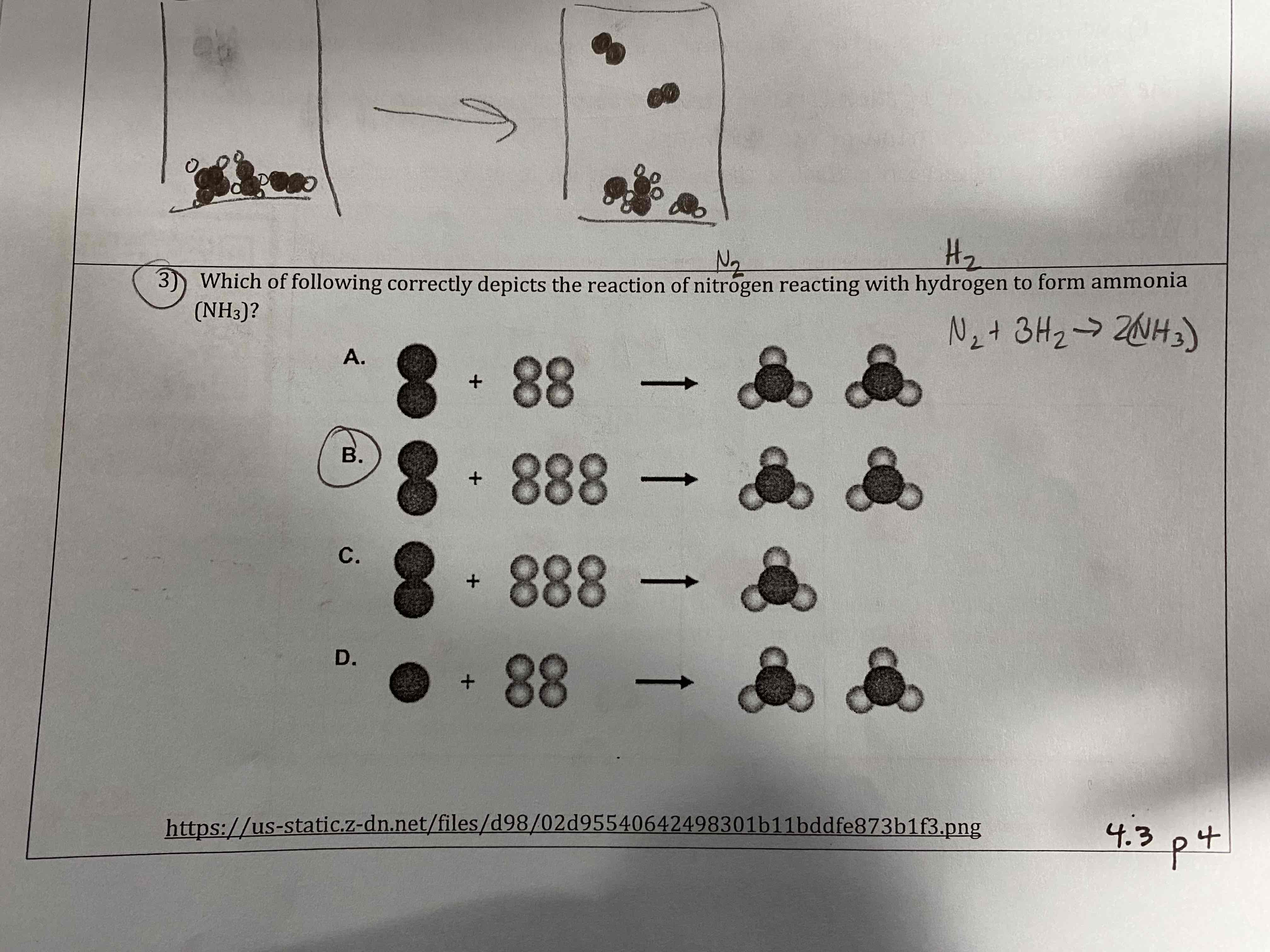
Which if the following correctly depicts the reaction of nitrogen reacting with hydrogen to form ammonia (NH3)?
B.
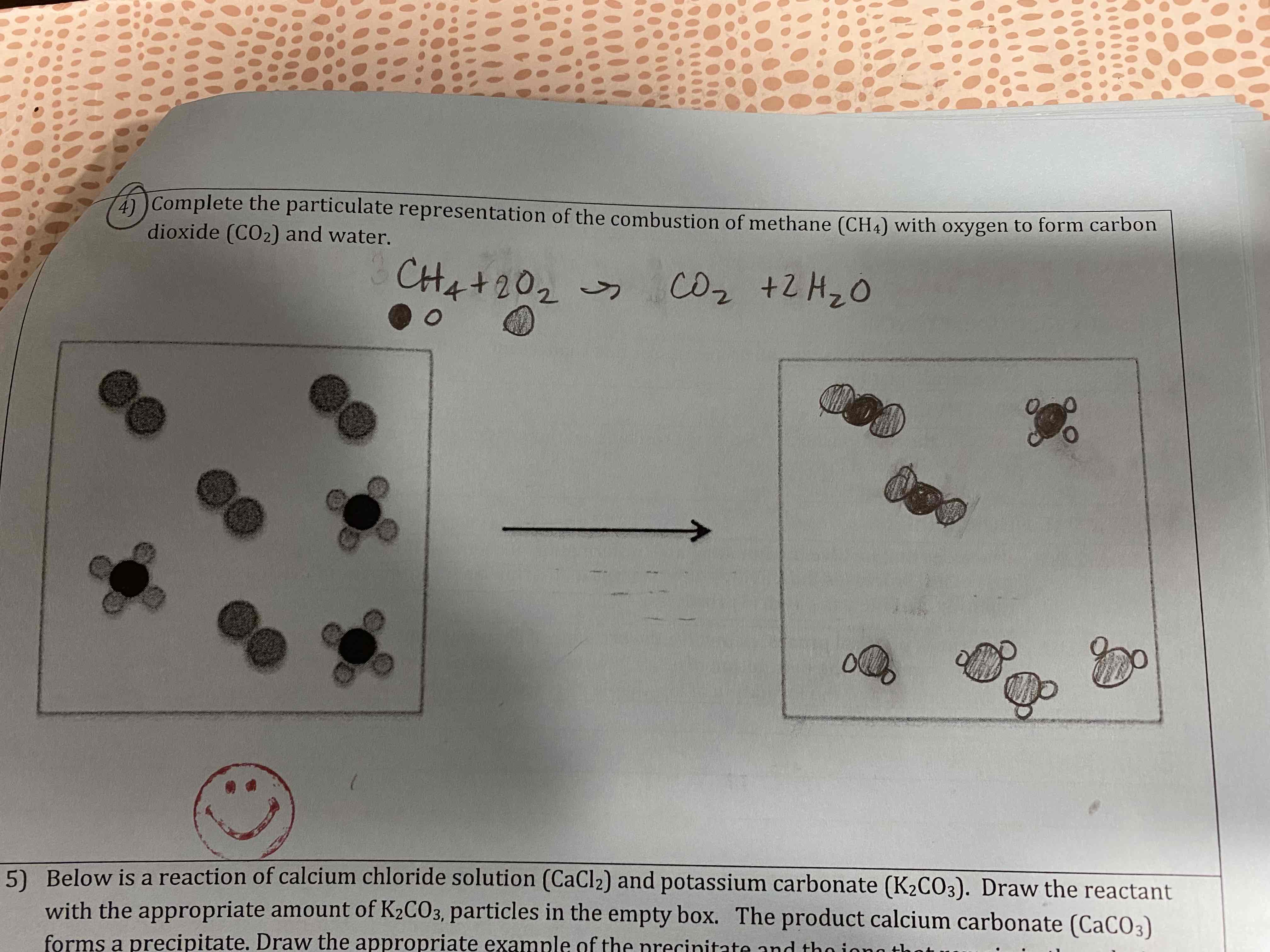
Complete the particulate representation of the combustion of methane (CH4) with oxygen to form carbon dioxide (CO2) and water.
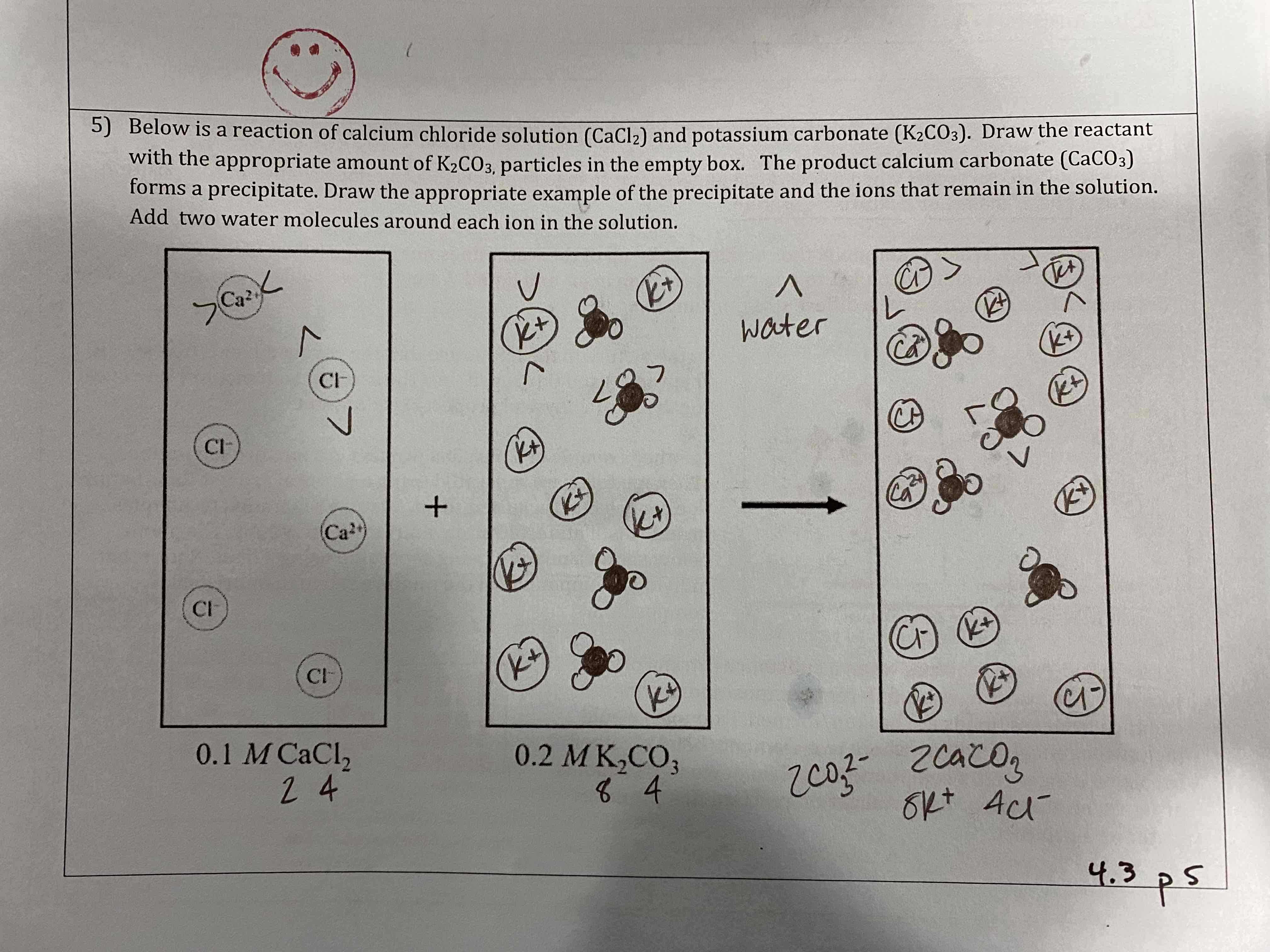
Below is a reaction of calcium chloride solution (CaCl2) and potassium carbonate (K2CO3). Draw the reactant with the appropriate amount of K2CO3 particles in the empty box. The product calcium carbonate (CaCO3) forms a precipitate. Draw the appropriate example of the precipitate and the ions that remain in the solution. Add two water molecules around each ion in the solution.
Label each process below as physical or chemical as well as the type of forced (intramolecular forces/intermolecular forces) broken/formed during the process.

Draw/Label a particle diagram of liquid HCl (hydrochloric acid) becoming a gas. Explain how this is a physical process vs a chemical process.

A student weighs a piece of metal and finds that it is 4.4 g. The student then holds this piece of metal with tongs and heats it using the flame of a Bunsen burner. After the final product is cooled, it is weighed and found to have a mass of 5.0 g. Which of the following statements related to the experiment is correct?
The mass of the metal increased when it went through a chemical change as bonds formed between the metal and another substance.
Which of the following represent chemical processes? Which represent physical processes?
a. Calcium chloride dihydrate slowly heated in a crucible to become calcium chloride.
b. A hydrocarbon such as propane undergoes combustion to power a grill.
c. A rock climber’s rope becomes frayed and turns the color of the rocks.
d. A dog urinates on an air conditioner coil and the coils become corroded.
a. Chemical
b. Chemical
c. Physical
d. Chemical
A student added methanol and dry sand together and noticed that bubbles emerged from the mixture when stirred until the mixture was completely wet. Do the bubbles produced indicate that a chemical reaction occurred?
No, air is escaping or being mixed into the methanol.
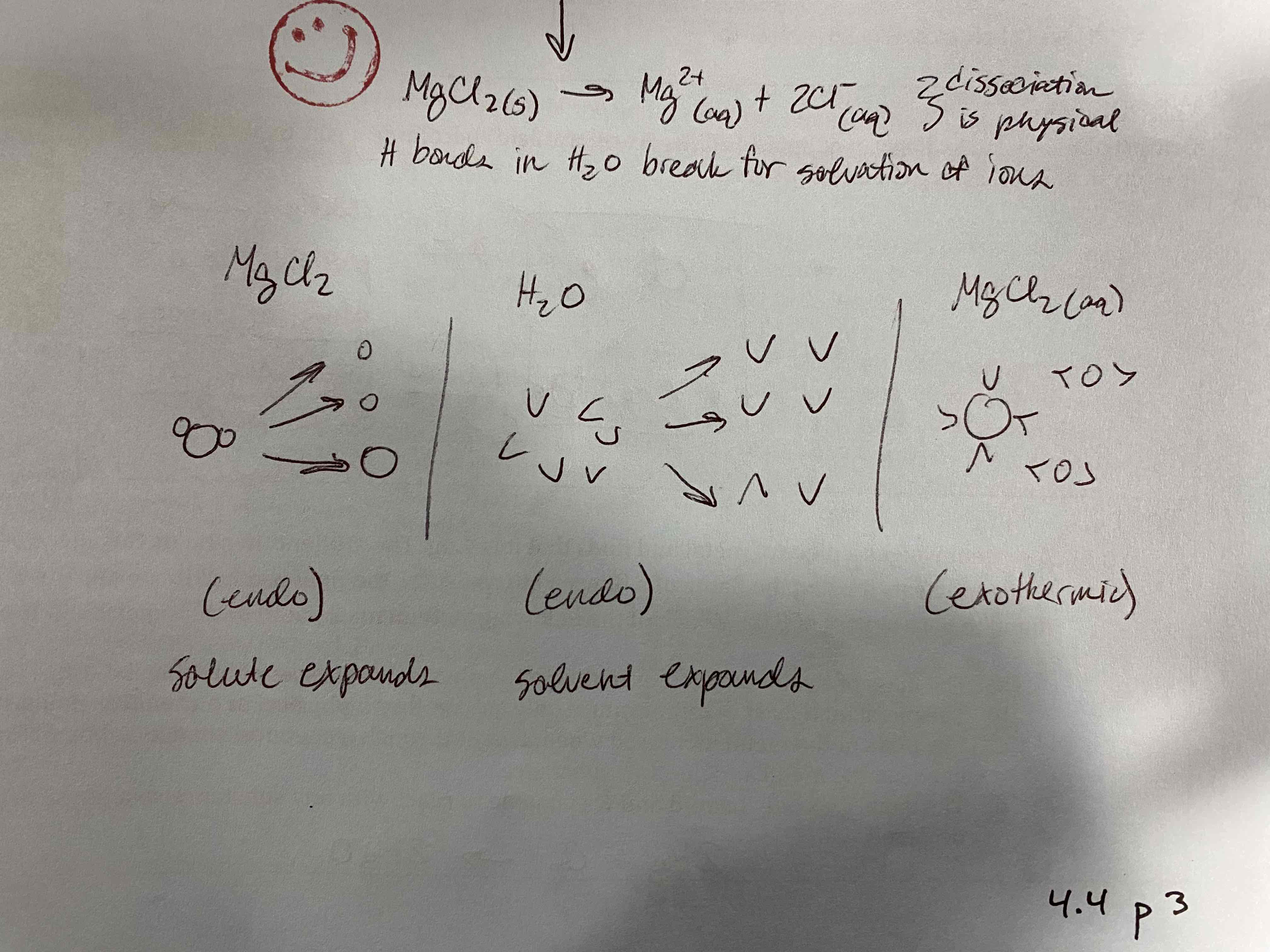
Physical processes involve the breaking of intermolecular forces and chemical processes involve the breaking of intramolecular forces. Draw/Label a particle diagram of MgCl2 dissolving in water showing how both processes are involved.
How many grams of lead product would theoretically be produced between 17.0 g potassium iodide, KI, and 25.0 g of lead (II) nitrate, Pb(NO3)2?
23.6 g PbI2

How much excess reaction is left over when 17.0 g of potassium hydroxide (KOH) reacts with 20.0 g iron (III) nitrate (Fe(NO3)3)?
3.05 g KOH

Medicinal “ether” is produced when ethyl alcohol is grated with an acid. How many grams of medicinal “ether” (C4H10O) would you produce with 50.0 g of ethyl alcohol (C2H6O) if the percent yield is 85%?
34.2 g C4H10O
