Cram Unit 3: Intermolecular Forces and Properties
1/48
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
Intermolecular forces
force between molecules
LDFs
nonpolar molecules or noble gases (atoms) that are able to form attractions through temporary and induced dipoles.
Polarizability can depend on
Size
Number of electrons
Contact area (depends on the structure)
LDF is a prerequisite to have
dipole-dipole interaction
Dipole-Dipole
attraction between permanent dipoles in polar molecules.
Hydrogen Bonding
H is covalently bonded directed to highly electronegative atoms N,O,F
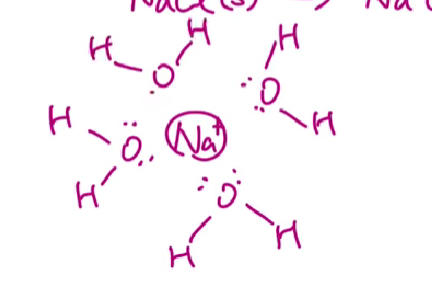
Ion-Dipole Force
Ionic compounds dissolved in water where the ions are attracted to the partial - and partial + ends of the dipole in the polar molecules.
Covalent Network Structure
The covalent bonds create a complex network structure.
High melting point and high boiling point
Rigid and hard
Only non-metals
Allotropes
different physical forms of the same element.
Carbon in covalent network solids
Ideal Gas Law
PV = nRT
P Pressure (atm)
V Volume (L)
n # of moles (mol)
R gas constant (0.0821)*
T temperature (K)
Ideal gas
Small size, big space, no intermolecular forces
Do not occupy much space given their size
Gas particles are far from each other
No attraction or repulsion

mol - K - atm - L constant
0.0821
mol - K - kPa - L constant
8.314
mol - L - mmHg - K constant
62.4
Pressure
Frequency of collision of gas particles in their container
Boyle’s Law
P1 V2 = P2 V2
At constant T, as pressure increase, volume decreases.
When volume decreases, the particles collide with the side of the container more often, thereby increasing pressure.
Charles’ Law
V1/T1 = V2/T2
At constant P, as volume increases, temperature increases.
When temperature or average kinetic energy increases, particles move faster causing more and stronger collisions with the walls of the container. The volume increases to keep the pressure constant.
Gay-Lussac's Law
Gay-Lussac’s Law
P1/T1 = P2/T2
At constant V, as pressure increases, temperature increases
As temperature increases, particles move faster causing collisions with the sides of the container to happen more often and to be stronger. This increases the pressure.
Combined Gas Law
P1V1/T1 = P2V2/T2
Avogadro’s Law
V1/n1= V2/n2
Adding more particles to a container causes more collisions with the walls of the container and the volume increases to keep the pressure constant
Partial pressure
the pressure applied by each of the gas particles in the container.
Example - P(total)= P(N2) + P(O2)
P(N2) = P(total) * n(N2)/n(total)
P(O2) = P(tota) * n(O2)/n(total)
Total pressure
the sum of the partial pressures
Real gases
these types of gases deviate from ideal gas law
Attraction is present because of IMFs
At high pressure, gas particles will gain more IMFs, making the deviation greater
At low temperature, gas particles will be more affected by IMFs, making the deviation greater.
If the size of the gas particles is bigger, they will form IMFs more easily, making the deviation greater.
Kinetic Molecular Theory
macroscopic properties of ideal gases due to the motion of particles.
Continuous random motion of the gas particles
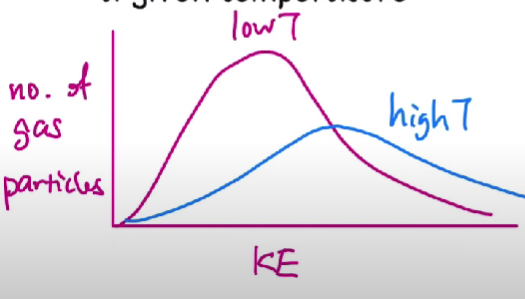
Maxwell-Boltzmann distribution
KE of particles at a given temperature.
KE= ½ m * v sqrd
Kinetic energy
In pink line, there are more gas particles that have a lower kinetic energy with a lower temperature while there a more particles with more kinetic energy in the blue line with a higher temperature.
Average kinetic energy
The energy of the entire batch of particles depends on temperature only.
Even if they are two different species (N2 and O2), if they are acting at the same temp. (300 K), then their average kinetic energy will be the same.
Average speed
If the particle has a greater molar mass, then they will have slower average speed.
If the particle has a lower molar mass, then they will have faster average speed.
This depends on the molar mass of the gas particles found in the sample, not the overall temperature.
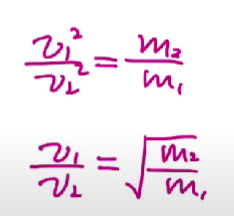
What does fixed temperature look like in speeds of particles?
Remember, all gas particles won’t have the same speed, just an average speed. So, if all particles look like they have the same speed, that’s wrong.
Solutions
A type of homogeneous mixture where pure substances are mixed in a uniform way. (You can’t tell the difference between different parts of the solution)
Can be solids, liquids, or gases
Concentration (M) (mol/L)
number of moles of the solute/volume of the solvent or solution (mol/L)
Concentration for gases
number of moles of the gas/volume of the container
Solubility and IMF
Substances with similar IMF tend to be soluble in one another.
Water and oil X (Hydrogen bonds and dipoles + LDFs)
Water and salt ! (Hydrogen bonds and dipoles - Ionic bonds → Ion-dipole bond)
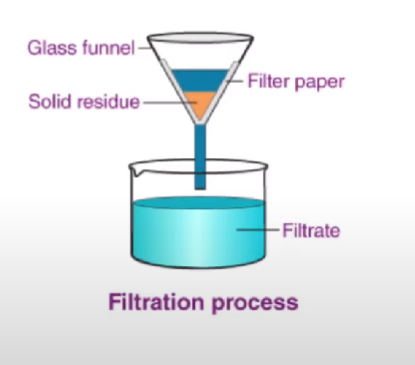
Filtration (Separating mixtures)
Used to separate heterogeneous mixture that is insoluble in water.
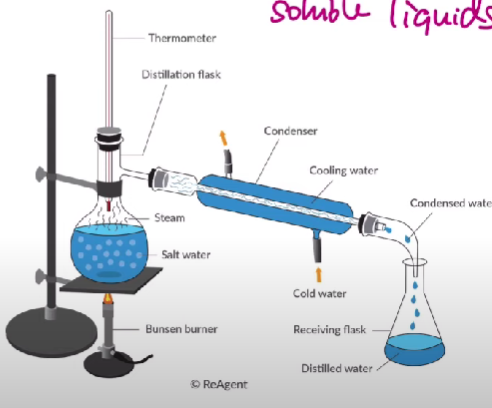
Distillation
Used to separate two soluble liquids from each other
Separates the solution with the lowest boiling point first because it uses heat.
Alcohol - 75°C
Water - 100°C
You’d raise the temperature to around 78°C, so the alcohol evaporates and passes through a condenser to become a condensed solution in the flask.
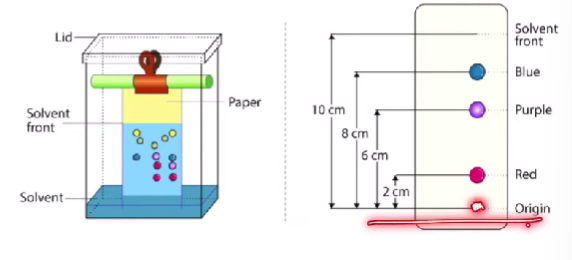
Chromatography
Paper chromatography → used to separate pure substances using a solvent
Depends on the size of the molecules
Depends on the polarity
E.g. (nonpolar solvent), the a nonpolar pure substance will rise up faster along with the solvent. A polar substance would not rise up as fast.
Bigger size - takes longer
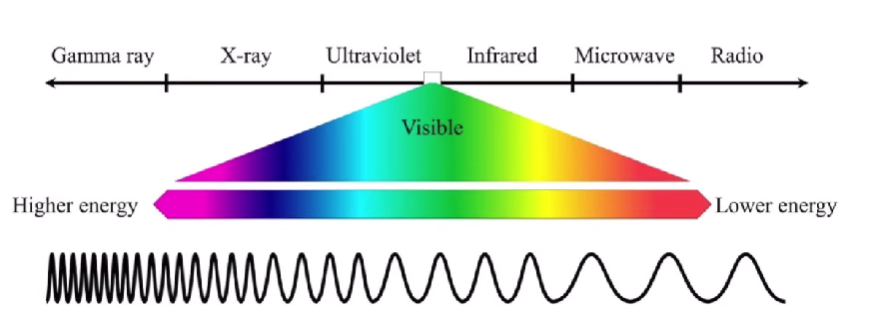
Electromagnetic spectrum
EM Radiation - types of light that behave like wave-particles.
Speed (electromagnetic spectrum)
C= 3 X 10 ‘8 m/s (THIS IS CONSTANT THROUGHOUT THE ELECTROMAGNETIC SPECTRUM)
Frequency
v (Hz, s-1)
Wavelength
λ (m, nm)
Speed of the electromagnetic radiation is equal to
λ * v (wavelength * frequency)
EM radiation
Highest v lowest λ Highest energy Gamma rays - X-ray - Ultraviolet - Infrared - Microwave - Radio Lowest v Highest λ Lowest energy
Photoelectric effect
Absorption of energy from light to eject electrons on the surface to become photoelectrons - One way is when light reaches the surface of a compound and ejects electrons from the surface if the energy carried by the photon is enough. These electrons being ejected become photoelectron as they absorb energy from the photons.
Depends on the frequency, aka the energy carried by the light particles.
Energy carried by photons
E = h v
h → Planck Constant (6.626 X 10 -3 J/s)
v → frequency
Molecular motion or electronic transition
Absorption of photons lead to different types of molecular motion or electronic transition.
Microwave radiation
Molecular rotational transitions
Infrared radiation
Molecular vibrational transitions
Ultraviolet radiation
Electronic energy level transitions (e- become excited and begin to move up and down the electron energy levels)
Spectrophotometry
Figure out the concentration of a certain solution using different light sources
Light can be absorbed by solutions depending on the concentration of the sample.
Solutions will absorb the opposite color that they are the most.
If there are externalities in the process such as more layers to go through in the cuvette, more light will be absorbed.
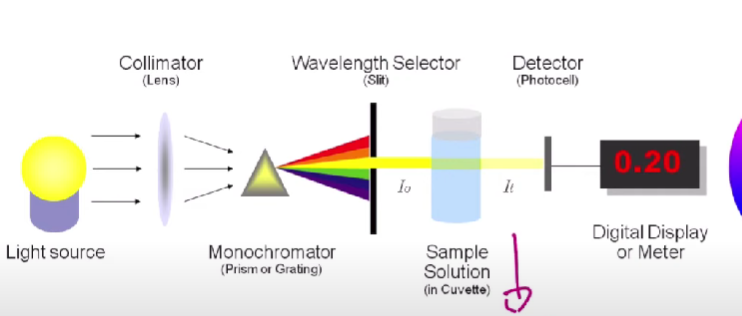
Beer-Lambert Law
A (absorbance) = a b c
a - absorptivity (how much a sample of the molecules or ions absorb light at specific wavelengths)
b - length of the cuvette
c - concentration (lighter vs darker color)
