ch 7: acetylcholine
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
25 Terms
acetylcholine synthesis mechanism (3)
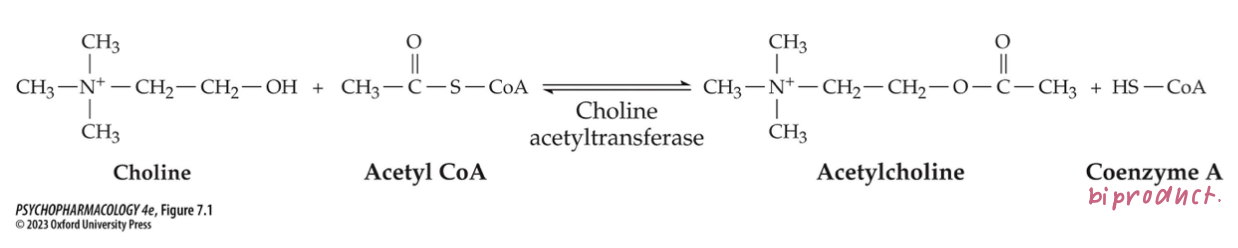
synthesized in one step from choline + acetyl coenzyme A (acetyl CoA)
catalyzed by choline acetyltransferase (ChAT)
rate of synthesis depends on availability of precursors + rate of cell firing → there are no inhibitors of ChAT
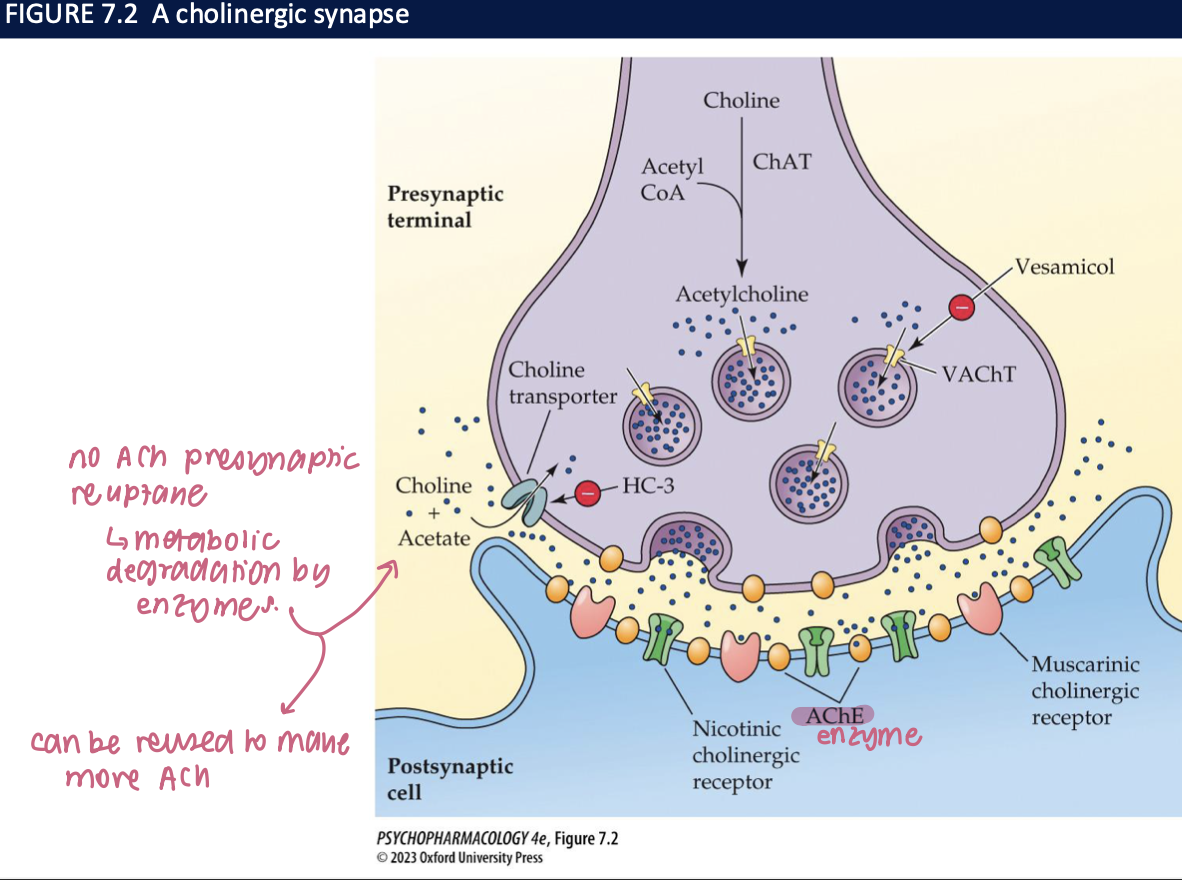
ACh is loaded into synaptic vesicles by _____ → which can be blocked by ____
vesicular ACh transporters (VAChT)
vesamicol → which reduces the amount of ACh released when neurons fire
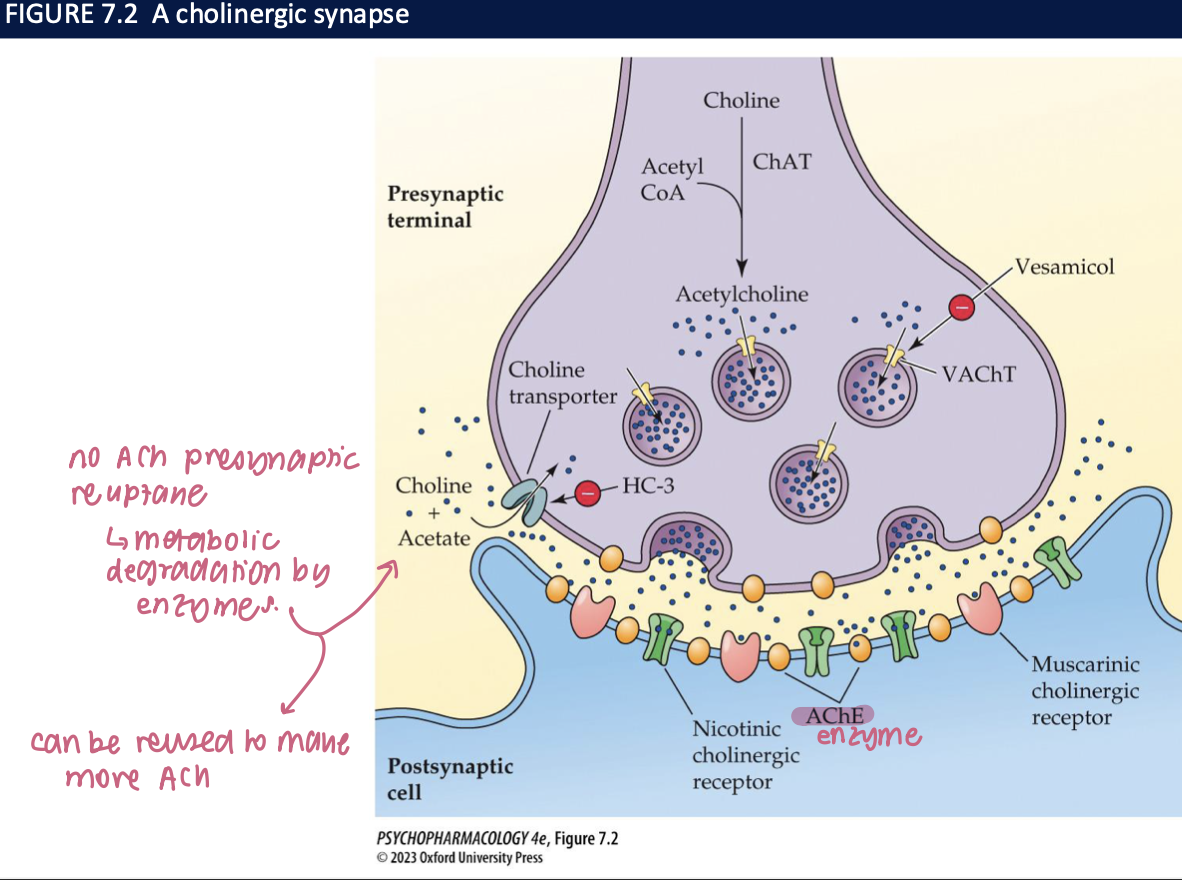
features of a cholinergic synapse
no ACh presynatic reuptake → metabolic degradation by enzyme
can be reused to make more ACh
examples of toxins that affect ACh release (2)
black widow spider venom causes massive release of ACh in the PNS
botulism toxin blocks ACh release by preventing fusion of synaptic vesicles w nerve terminal membrane → causes muscular paralysis
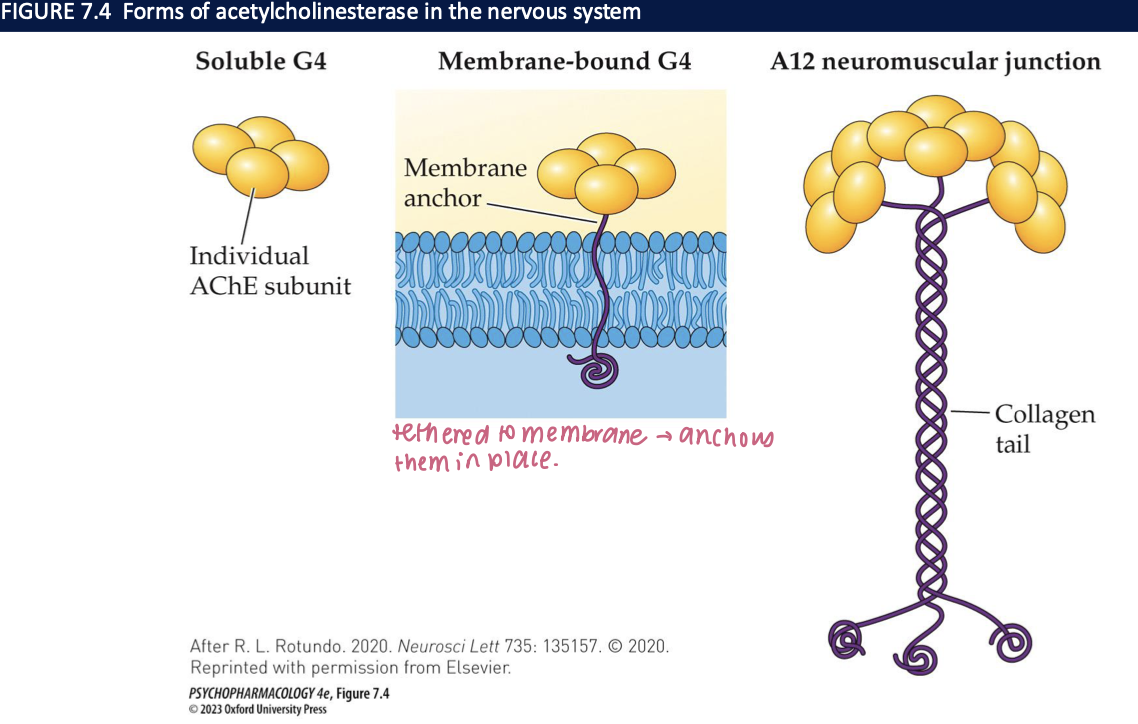
acetycholinesterase (AChE) (4)
breaks ACh down to choline + acetic acid → four subunits combine to form a tetramer (G4)
3 forms:
soluble: free G4 in the cell
G4 tethered to the cell membrane in a position to allow rapid breakdown
A12 → at neuromuscular junctions → 12 subunits v. rapid breakdown of ACh allows muscle to relax before next contraction

why is recycled choline critical in maintaining ongoing ACh synthesis? + how do we know? (3)
choline taken back up into the cholinergic nerve terminal by a choline transporter + used to synthesize more choline
if choline transporter blocked (ie. using hemicholinium-3, HC-3) → ↓rate of ACh production
choline transporter KO mice: die after 1 hour of birth bc failure to sustain ACh synthesis + release at neuromuscular junction
AChE inhibitors — reversible vs irreversible, examples & key uses/toxicity (5)
Reversible (↑ ACh; shorter-acting):
Donepezil, rivastigmine, galantamine → Alzheimer’s/dementia (CNS-active).
Physostigmine (crosses BBB) → glaucoma (topical; systemic = toxic).
Pyridostigmine → myasthenia gravis; also prophylaxis against nerve gas (temporarily occupies AChE to prevent permanent inactivation).
Irreversible (organophosphates; long-acting):
Insecticides and nerve agents (e.g., sarin, soman) → severe cholinergic excess; potentially fatal.
Toxicity theme: too much ACh → DUMBBELSS (diarrhea, urination, miosis, bronchospasm, bradycardia, emesis, lacrimation, sweating, salivation).
🧠 Takeaway: Reversible AChE inhibitors are therapeutic (memory, MG, glaucoma); organophosphates are dangerous poisons.
myasthenia gravis + treatment (3)
autoimmune disorder in which antibodies against muscle cholinergic receptors are produced
leads to severe muscle weakness + fatigue bc muscles less sensitive to ACh
treated w Neostigmine + pyridostigmine → don’t cross the BBB so only affect the periphery
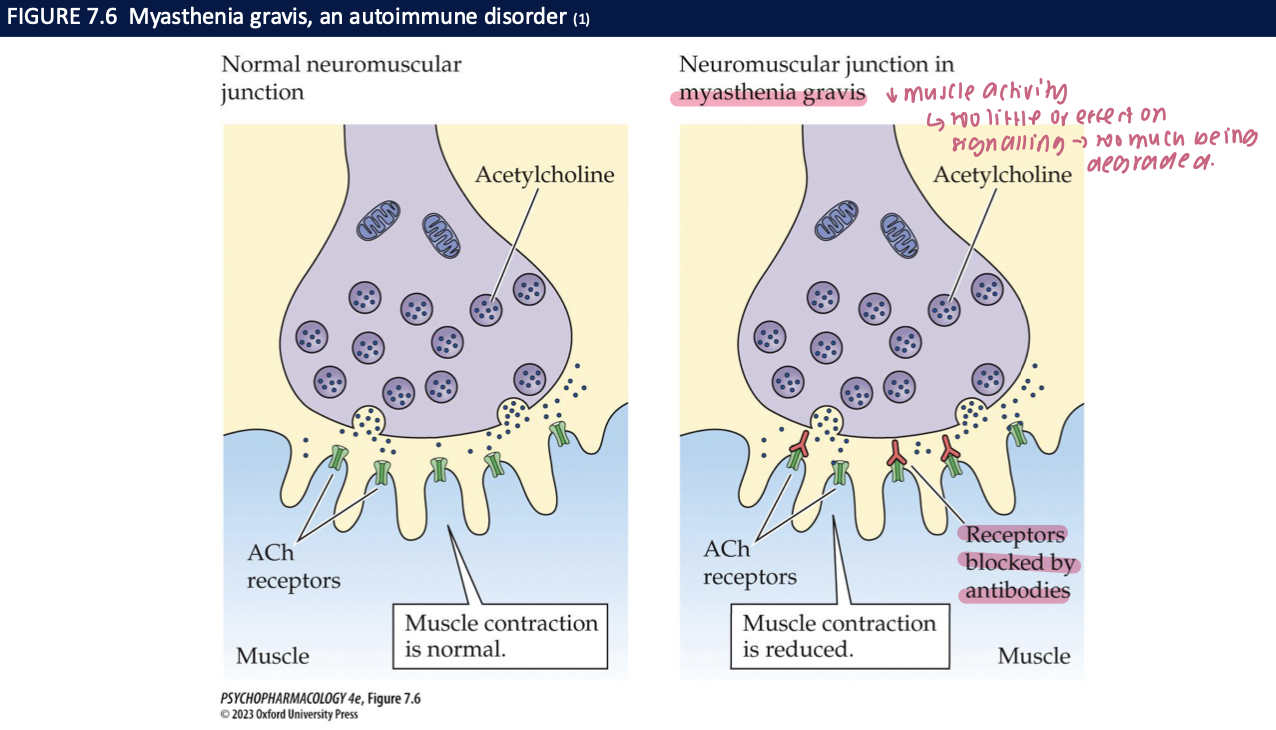
myasthenic syndromes arise from mutations that encode for ___ or ___
ChAT: ↓ChAT = insufficient amounts of ACh are released at NMJ
AChE: deficient AChE = persistently elevated lvls of ACh at NMJ lead to desensitization of receptors + reduced cholinergic transmission
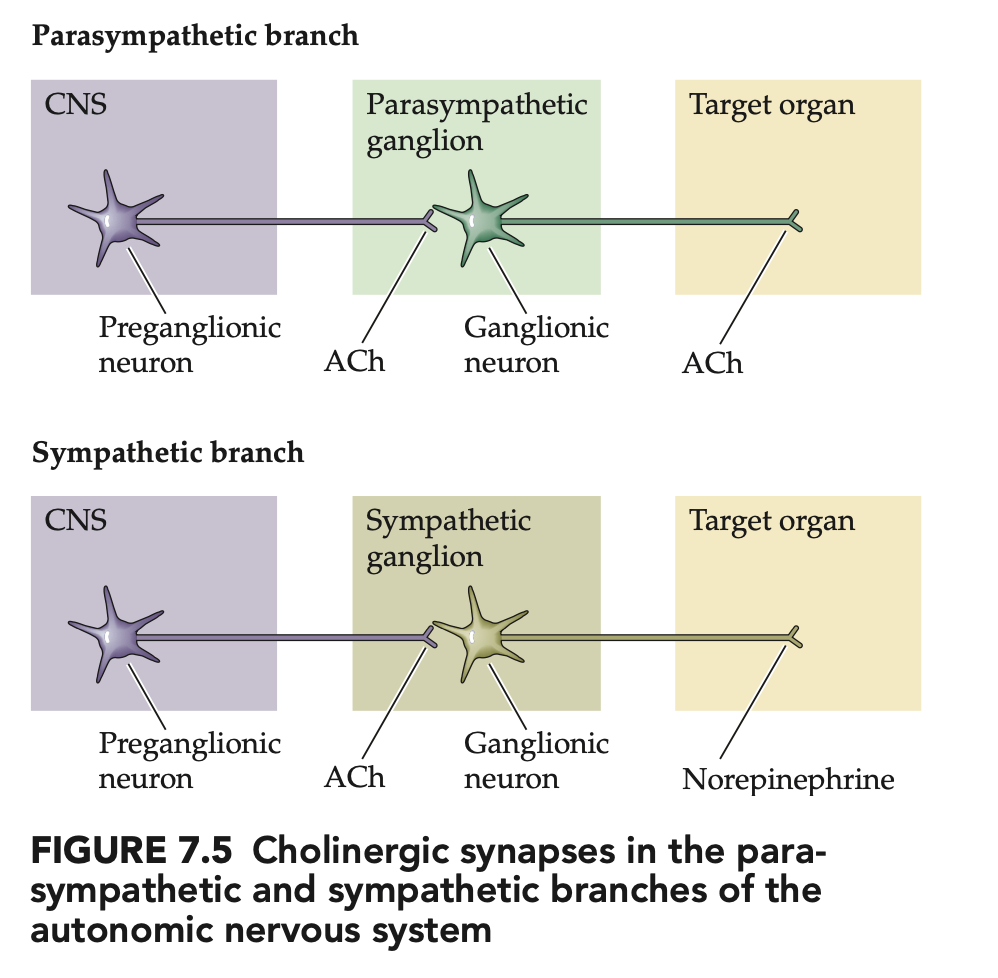
Which parts of the ANS use ACh vs NE? (4)
Preganglionic neurons (symp + parasymp): ACh (nicotinic)
Parasympathetic postganglionic: ACh (muscarinic) to target organs
Sympathetic postganglionic: mainly NE to targets (classic exception: sweat glands = ACh)
🧠 Takeaway: ACh is everywhere preganglionic; parasymp is cholinergic, symp is mostly noradrenergic.
What is the basal forebrain cholinergic system (BFCS) and where does it project? (2)
Nuclei: Medial septum (MS), diagonal band (DBB), nucleus basalis / substantia innominata (NB/SI)
Projections: MS/DBB → hippocampus; NB/SI → neocortex/PFC + limbic cortex
🧠 Takeaway: BFCS provides the major cholinergic input to hippocampus and cortex.
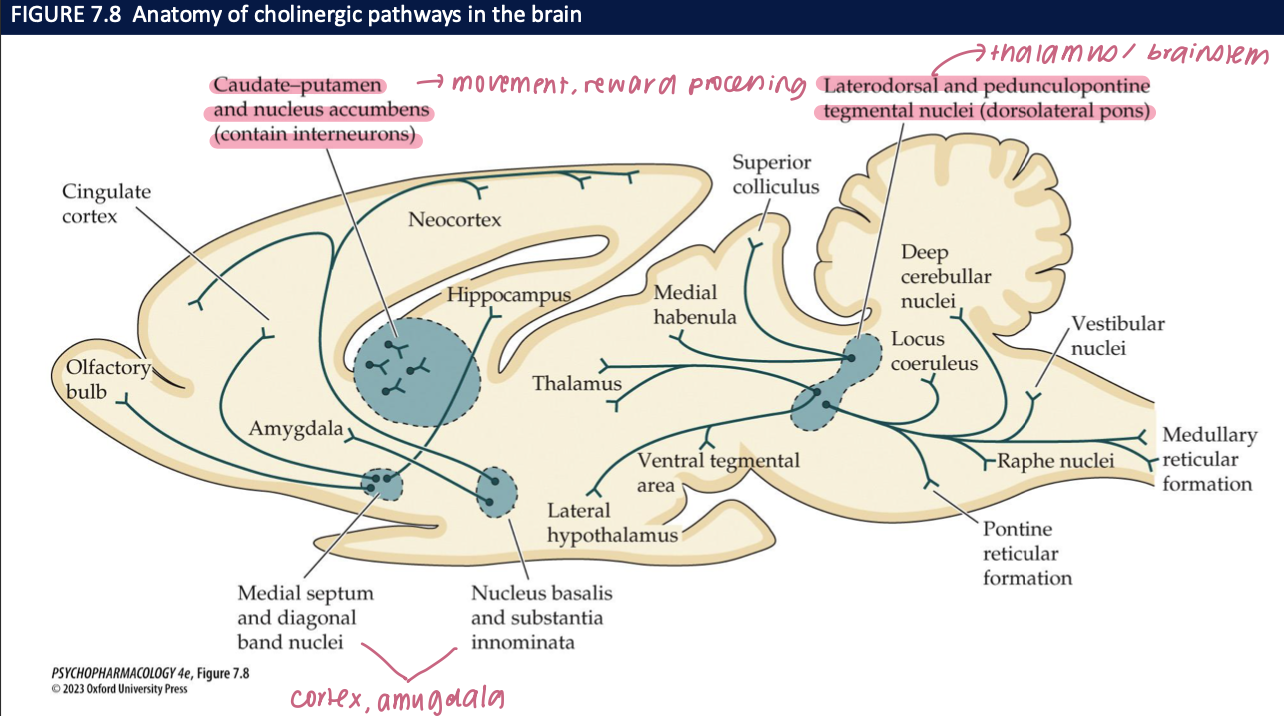
ACh & cognition—what functions map to which BFCS pathways? (3)
MS/DBB → hippocampus: encoding of declarative memory
NB/SI → PFC/cortex: sustained attention & cue detection
Optogenetics: Bursts of ACh release improve detection of learned sensory cues; inhibition impairs
🧠 Takeaway: Hippocampus = memory, PFC/cortex = attention, and phasic ACh boosts performance.
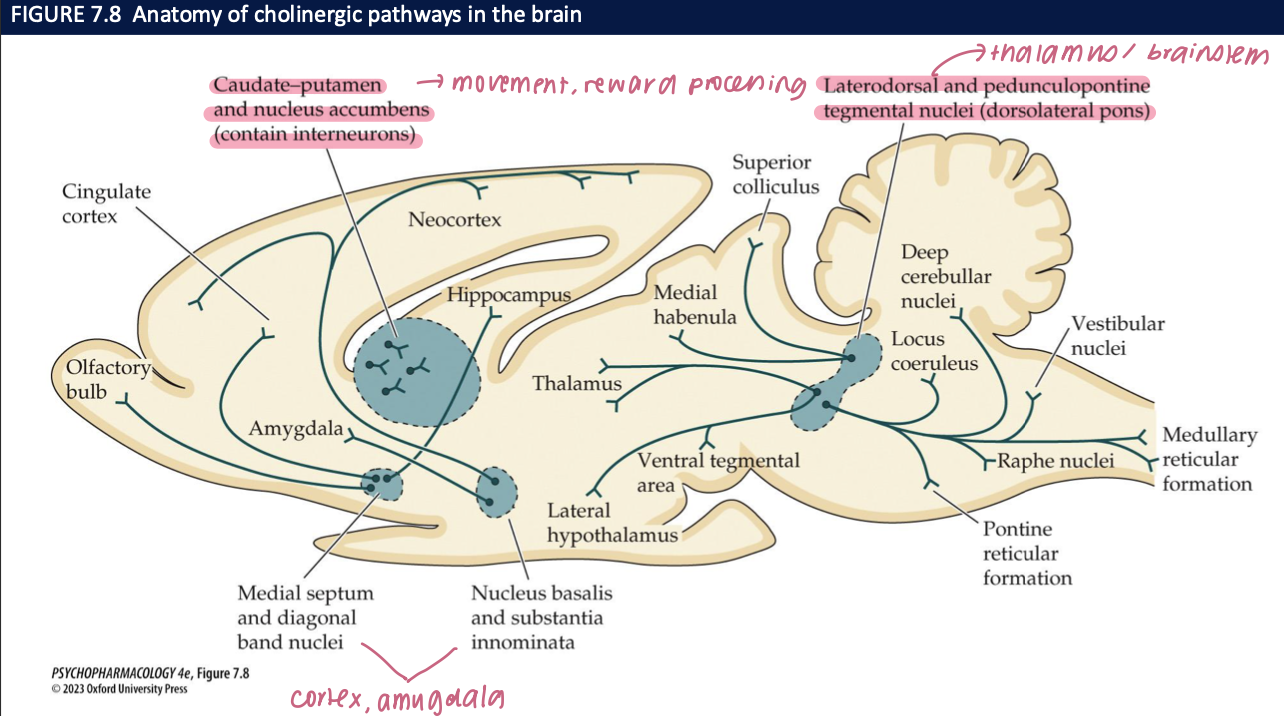
Where are brain cholinergic cell bodies concentrated, and what’s the big-picture anatomy? (2)
Clusters rather than diffuse: BFCS (MS/DBB/NB/SI) + brainstem cholinergic nuclei
Projections are widespread to cortex, hippocampus, amygdala, thalamus → modulate arousal, learning, attention
🧠 Takeaway: Small nuclei, huge reach—ACh broadcasts to many cognitive networks.
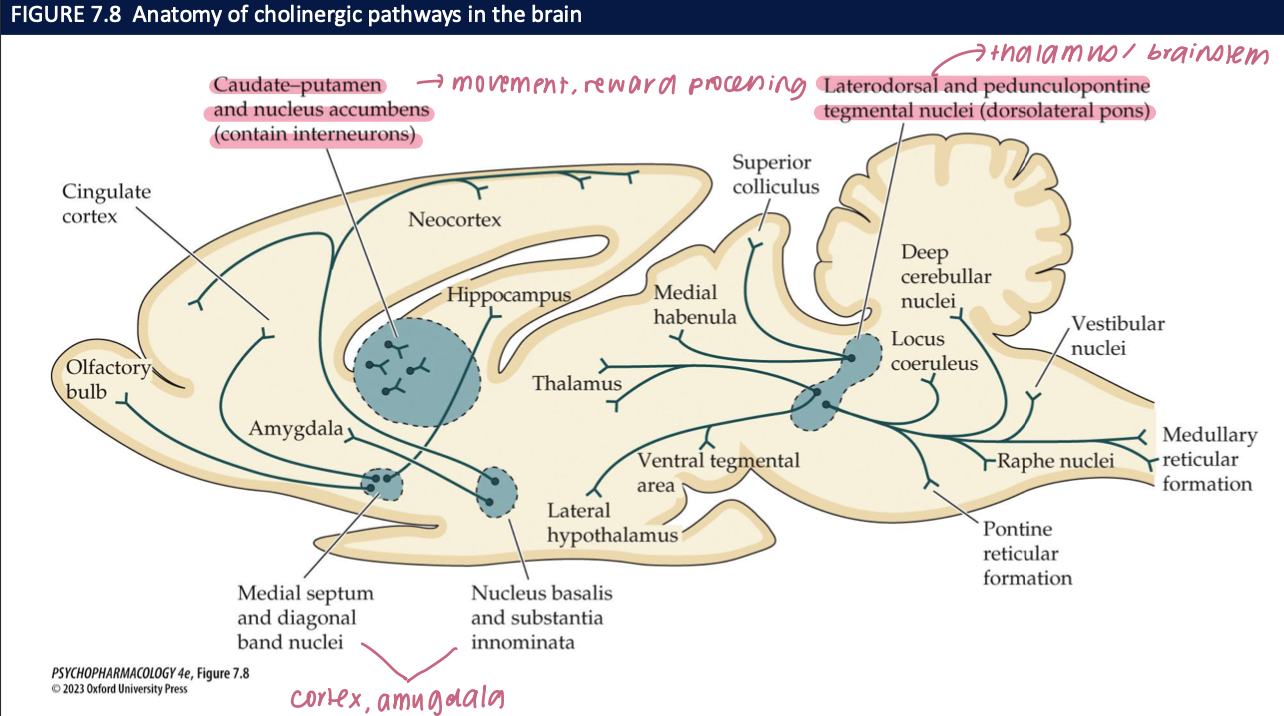
How does ACh interact with DA in the striatum, and why does it matter in Parkinson’s? (2)
Striatal interneurons (ACh) balance dopamine to regulate movement
Parkinson’s: low DA → transmitter imbalance; anticholinergic drugs can reduce tremor/rigidity in some patients
🧠 Takeaway: ACh–DA balance is key for motor control; tipping toward ACh contributes to Parkinsonian signs.
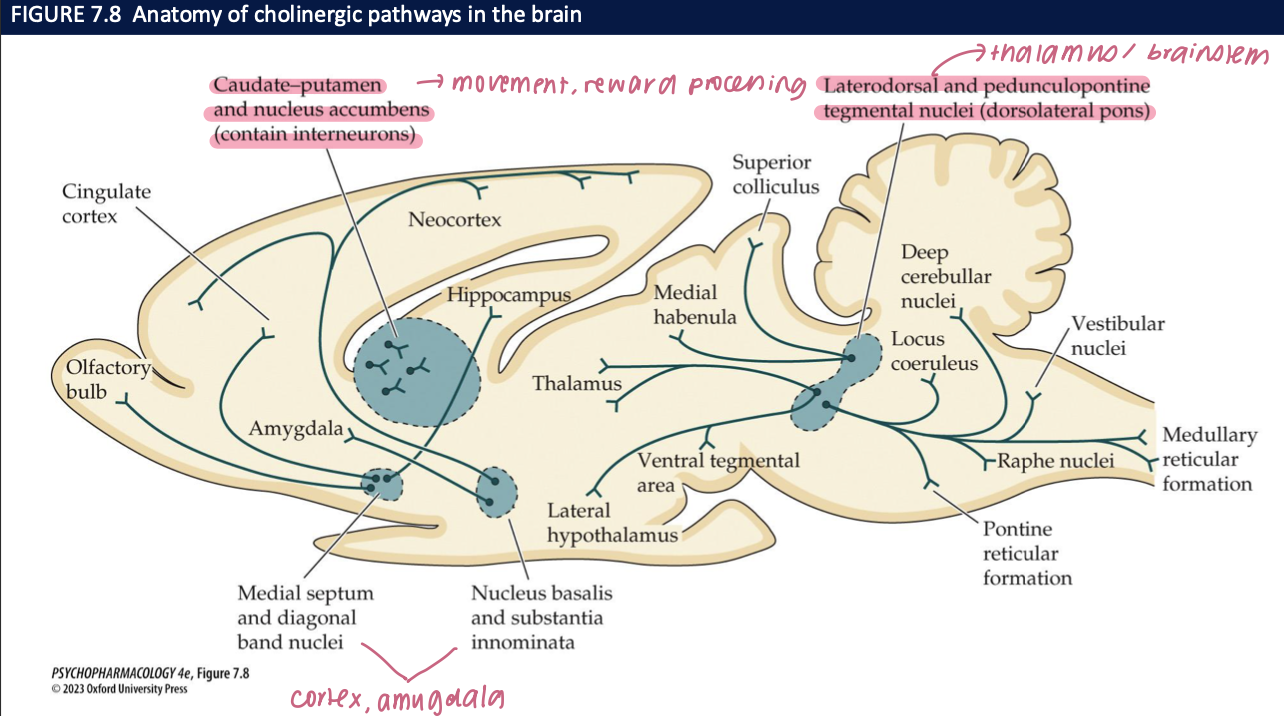
what do the effects of optogenetic stimulation/inhibition of basal forebrain cholinergic system (BFCS) neurons on visual cue detection show about basal forebrain ACh and attention? (4)
Setup: Optogenetic phasic stimulation or inhibition of BFCS cholinergic neurons during a brief sensory cue (25 ms vs 500 ms).
Result (Stim): ACh bursts ↑ hit rate, especially at 25 ms cues (harder signals).
Result (Inhib): ACh inhibition ↓ hits, most at 25 ms.
Conclusion: Phasic ACh causally improves cue detection / sustained attention; removing ACh impairs it.
🧠 Takeaway: ACh boosts detection of brief signals—it’s not just correlated with attention; it drives it.
what do LDTg/PPTg cholinergic nuclei do, and where do they project? (2)
Projections: to thalamus & basal ganglia (arousal/sensory gating), to VTA/SNc DA neurons (↑ DA firing → reward/reinforcement), and down to brainstem/spinal cord (initiate REM sleep)
Role: brainstem ACh that links arousal + reward + REM
🧠 Takeaway: LDTg/PPTg ACh drives attention, primes dopamine, and triggers REM.
two types of ACh receptors
nicotinic receptors → respond to the agonist nicotine
ionotropic: mediate fast, excitatory responses in CNS/PNS
metabotropic receptors → respond to
operate via 2nd messengers
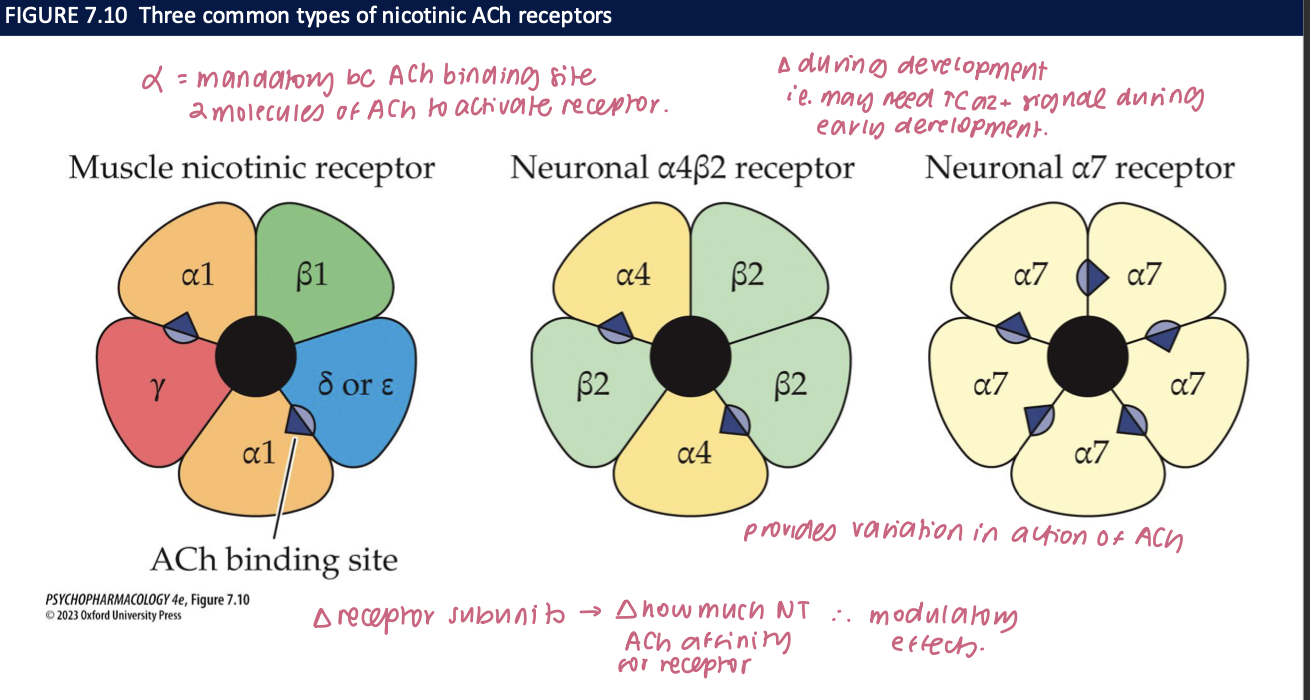
nicotinic receptors (nAChRs) (5)
ACh binds → the channel opens → Na+ and Ca2+ enter the neuron/muscle cell = depolarizes cell membrane
has 5 subunits → α, ß, γ, δ, ε
α: mandatory unit bc ACh binding site
2 molecules of ACh to activate receptor
∆ subunits = ∆ ACh affinity for receptor → modulatory effects
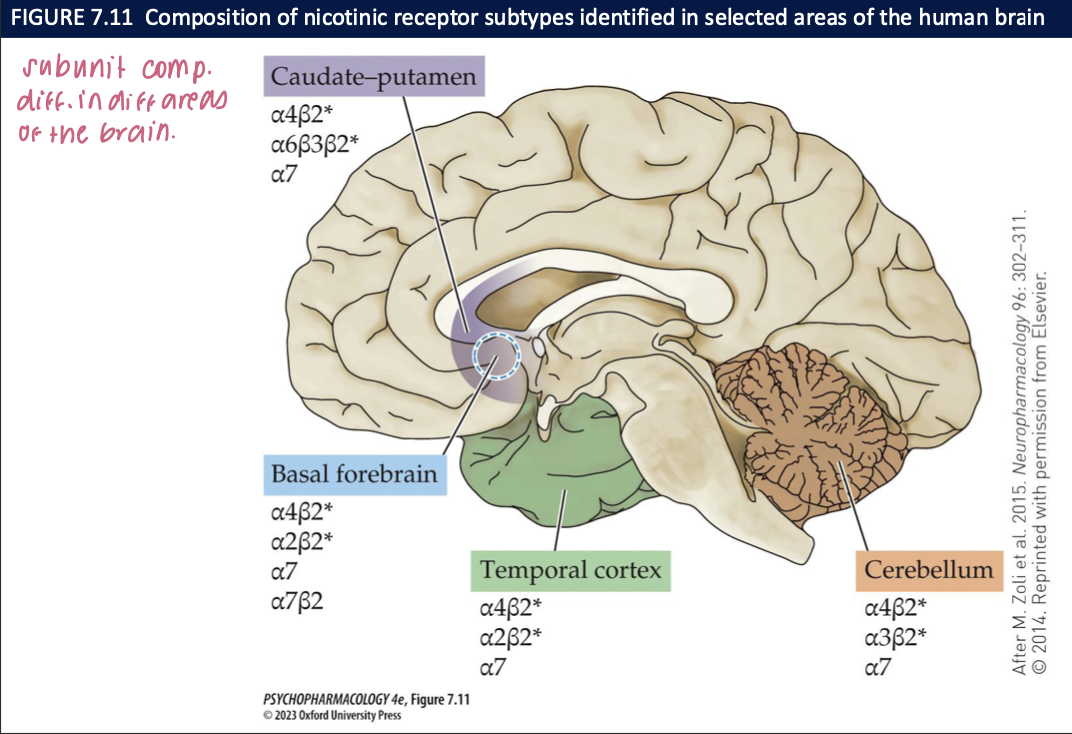
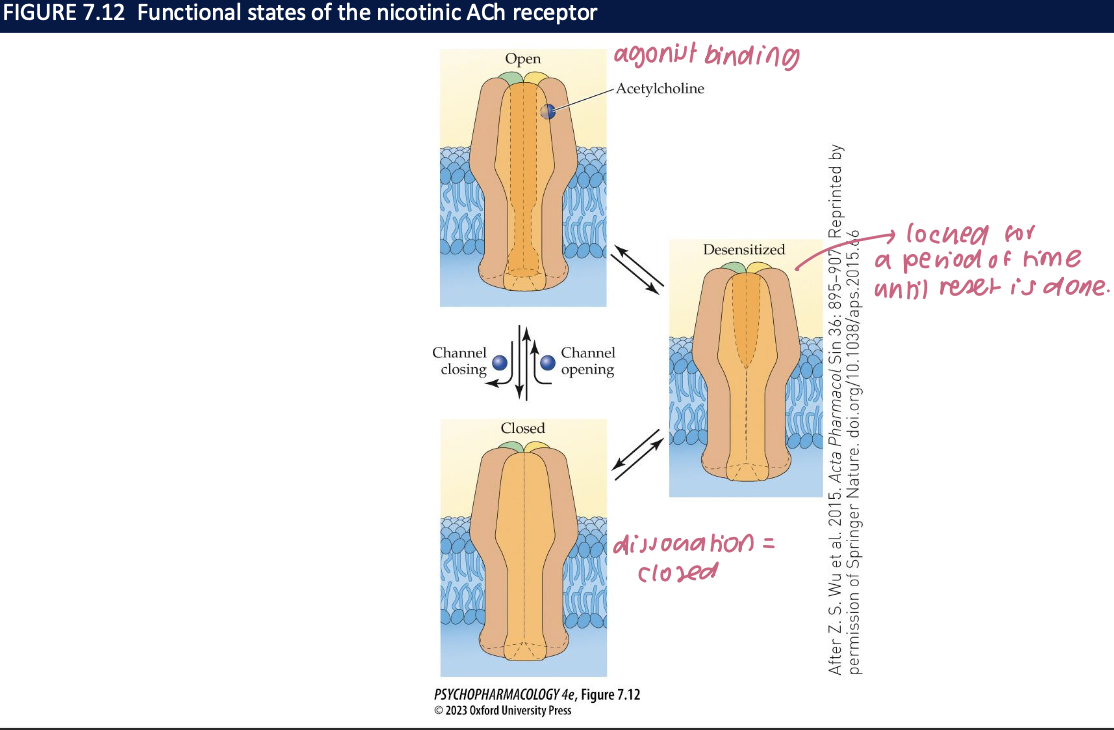
ionotropic channels can be ___, open, or _____ (channel is ___ + ____)
closed
desensitized
closed
won’t open even if an agonist binds
prolonged exposure to an agonist enhances the rate of conversion to the desensitized state → can spontaneously resensitize
metabotropic receptors subtypes (3)
5 subtypes
M1, M3, M5: activate PIP2 messenger system → excitatory
M2 + M4: inhibit cAMP synthesis → stimulate K+ channel opening + inhibit nerve terminal Ca2+ channel opening → hyperpolarization
depolarization block
resting potential of the membrane is lost + cell cannot be excited until agonist is removed + membrane repolarized
in the brain, muscarinic receptors are found in (3)
neocortex + hippocampus → cognitive effects of ACh
striatum → involved in motor function
M5 in brain is expressed primarily in the hippocampus, hypothalamus, and midbrain DA areas → may have a role in drug reinforcement
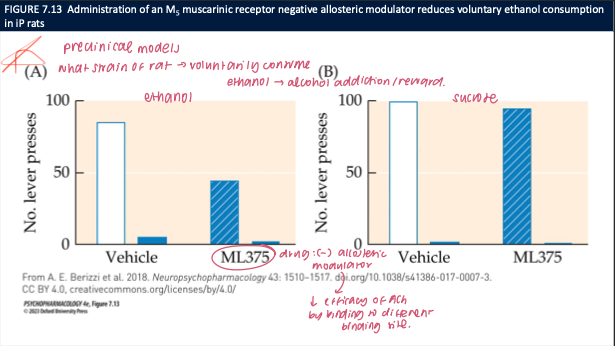
what does ML375 (M5 muscarinic NAM) do to ethanol vs sucrose self-administration in P rats, and what’s the takeaway? (3)
ML375 ↓ ethanol lever presses; no effect on sucrose responding
Acts as a negative allosteric modulator at M5, dampening ACh-M5 signaling
🧠 Takeaway: M5 receptors facilitate alcohol reward (likely via DA); M5 inhibition selectively reduces ethanol seeking without general suppression of reward.
in the PNS, muscarinic receptors are found in ___ + effect of drugs (2)
cardiac + smooth muscle → activated by ACh relased from parasympathetic postganglionic fbres
drugs used to treat many psychiatric disorders block peripheral muscarinic receptors especially in secretory glands → causes dy mouth effect
naturally occurring muscarinic receptor agonists/antagonists (4)
agonists: muscarine, locarpine, arecoline (parasympathomimetic agents)
mimic effects of parasympathetic activation → high doses can be fatal
antagonists: atropine, scopolamine (found in henbane + deadly night shade)
inhibit parasympathetic action (parasympatholytic agents)