principles of chemistry
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
Mixture
One or more pure substance
Homogeneous
Master is a uniform in appearence and has the same properties throughout
Percent
Out of 100
Heterogeneous
Matter Consist of two or more Physically distinct Phases
Mass
Amount of matter in an object
Fill in the blank: The amount of matter in an object is called ______.
Mass.
Weight
Effect of gravity of an object
Thermal energy
A form of energy involving the motion of small particles of matter
Temperature
Measure of the intesity of thermal energy of a system
Heat
The flow of energy due to a tempreture difference ( flows from regions of higher to lower temperature )
Density(d)
The ratio of the mass of a Substance to ik volume occupied by the mass
To get Celsius
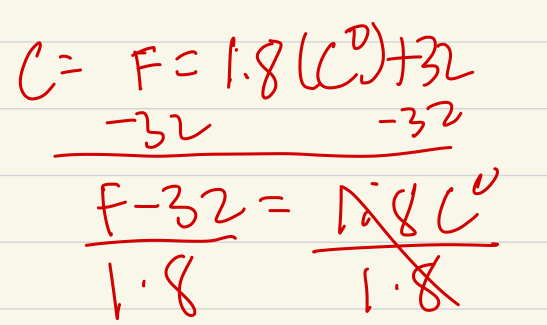
Fill in the blank: ______ is a form of energy involving the motion of small particles of matter.
Thermal energy
Fill in the blank: A ______ mixture is uniform in appearance and has the same properties throughout.
Homogeneous
Fill in the blank: A ______ mixture consists of two or more physically distinct phases.
Heterogeneous
Atoms
The basic unit of a chemical element, consisting of a nucleus surrounded by electrons.
Fill in the blank: The basic unit of a chemical element is called ______.
An atom.
Ionic compound
A metal element with a non-metal element ex: NaCl sodium chloride
Molecular compound
A non-metal element with a non-metal element ex: h2o
The law of conservation energy
States that energy can be created nor destroyed
Melting
solid to liquid
Freezing
Liquid to solid
boiling
liquid to gas
condensation
gas to liquid
sublimation
solid to gas
deposition
Gas to solid
Specific heat
The amount of heat required to raise the temperature of one gram of a substance by one degree Celsius.
what do chemical reactions do
absorb or release energy
what can chemical changes do
produce different kinds of energy like electricty energy
how much energy is in one calorie
4.184J
what is the Sl unit for energy
Joule(J)
Combositon of gasoline
chemical
sawing of wood
physical
Law
A summary of observed behavior
A theory or model
an attempt to explain the obervation behavior