ACID AND BASE
1/26
Earn XP
Description and Tags
All from our topic it’s include in the text passage
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
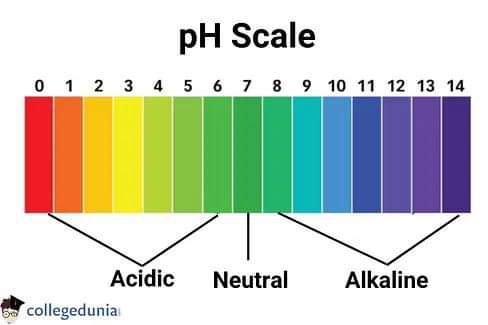
What is the pH scale?
The pH scale is a measure of the acidity or basicity of a solution, ranging from 0 to 14, where 7 is neutral, below 7 is acidic, and above 7 is basic
Give an example of a strong acid.
Hydrochloric acid (HCl) is an example of a strong acid.
Give an example of a strong base.
Sodium hydroxide (NaOH) is an example of a strong base
What is the chemical formula of the bicarbonate ion?
The chemical formula of the bicarbonate ion is HCO₃⁻.
What is an acid?
An acid is a substance that donates protons (H⁺ ions) in a chemical reaction.
What is a base?
A base is a substance that accepts protons (H⁺ ions) in a chemical reaction.
What is the chemical formula for sulfuric acid?
The chemical formula for sulfuric acid is H₂SO₄.
Define a Lewis acid.
A Lewis acid is a substance that can accept a pair of electrons.
Define a Lewis base.
A Lewis base is a substance that can donate a pair of electrons.
What is the Arrhenius definition of an acid?
According to the Arrhenius definition, an acid is a substance that increases the concentration of hydronium ions (H₃O⁺) when dissolved in water.
What is a conjugate acid-base pair?
A conjugate acid-base pair consists of two species related by the loss or gain of a proton.
What is the formula for the conjugate acid of the hydroxide ion (OH⁻)?
The conjugate acid of the hydroxide ion is water (H₂O).
What is the formula for the conjugate base of the hydronium ion (H₃O⁺)?
The conjugate base of the hydronium ion is the hydroxide ion (OH⁻).
What is the pH of a neutral solution?
The pH of a neutral solution is 7.
What is the relationship between pH and [H⁺] concentration?
pH is the negative logarithm of the hydrogen ion concentration ([H⁺]).
What is the pH of a solution with a [H⁺] concentration of 1 x 10⁻⁵ M?
The pH of a solution with a [H⁺] concentration of 1 x 10⁻⁵ M is 5.
What is the role of buffers in a solution?
Buffers help to resist changes in pH by accepting or donating protons as needed.
Define the term "acid dissociation constant (Ka)."
The acid dissociation constant (Ka) is a measure of the strength of an acid in solution, indicating the extent to which the acid dissociates into its ions.
Define the term "acid dissociation constant (Ka).
The acid dissociation constant (Ka) is a measure of the strength of an acid in solution, indicating the extent to which the acid dissociates into its ions.
Describe the Bronsted-Lowry theory of acids and bases.
According to the Bronsted-Lowry theory, an acid is a proton
What is the role of indicators in acid-base titrations?
Indicators are used to visually signal the endpoint of an acid-base titration by changing color at a specific pH.
What is the pKa value?
The pKa value is the negative logarithm of the acid dissociation constant (Ka), providing a measure of the strength of an acid. Lower pKa values indicate stronger acids
Explain the concept of acid strength.
A_d strength refers to the extent to which an acid ionizes or donates protons in solution. Strong acids ionize completely in solution, while weak acids ionize only partially.
What is the Henderson-Hasselbalch equation used for?
The Henderson-Hasselbalch equation is used to calculate the pH of a buffer solution based on the concentrations of the weak acid and its conjugate base (or vice versa).
Define the term "acid-base titration."
Acid-base titration is a laboratory technique used to determine the concentration of an acid or a base in a solution by neutralizing it with a solution of known concentration.
What is the pH range of basic solutions?
The pH range of basic solutions is above 7.
Describe the concept of base strength.
Base strength refers to the ability of a base to accept protons in solution. Strong bases fully dissociate into ions in solution, while weak bases only partially accept protons.