Introduction to Organic Chemistry and IUPAC Nomenclature
organic chemistry- chemistry concerning carbon-containing compounds
Why is Carbon Special?
- can form strong bonds with multiple atoms of itself in a chain
- can bond with many other elements like hydrogen, nitrogen, sulfur, oxygen, and phosphorus
- brings diversity in compounds that can be formed
- this allows it to be the basis of life
- carbon can also form double and triple bonds
- carbon is tetravalent- it can remove/gain 4 electrons
- functional groups- increase the functionality/reactivity of a molecule
Other Elements
- oxygen is divalent- it can gain 2 electrons
- nitrogen is trivalent- it can gain 3 electrons
- hydrogen is always monovalent- it can lose 1 electron
Types of Structures/Formulas & Other Conventions
lewis structures- show lone pairs as well as number & type of bond present in the molecule
condensed formula- writing the carbons with their hydrogens
- example- C20H42O can also be written as CH3(CH2)19OH
**bond line formula- **shown below
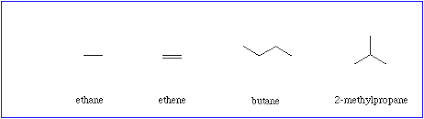
n-(insert molecular formula or name of hydrocarbon) means it’s “normal” & not branched
- example- *n-*butane
isomers- molecules with the same molecular formula but different structures
- the number of isomers tends to increase as the number of carbons in the compound increases
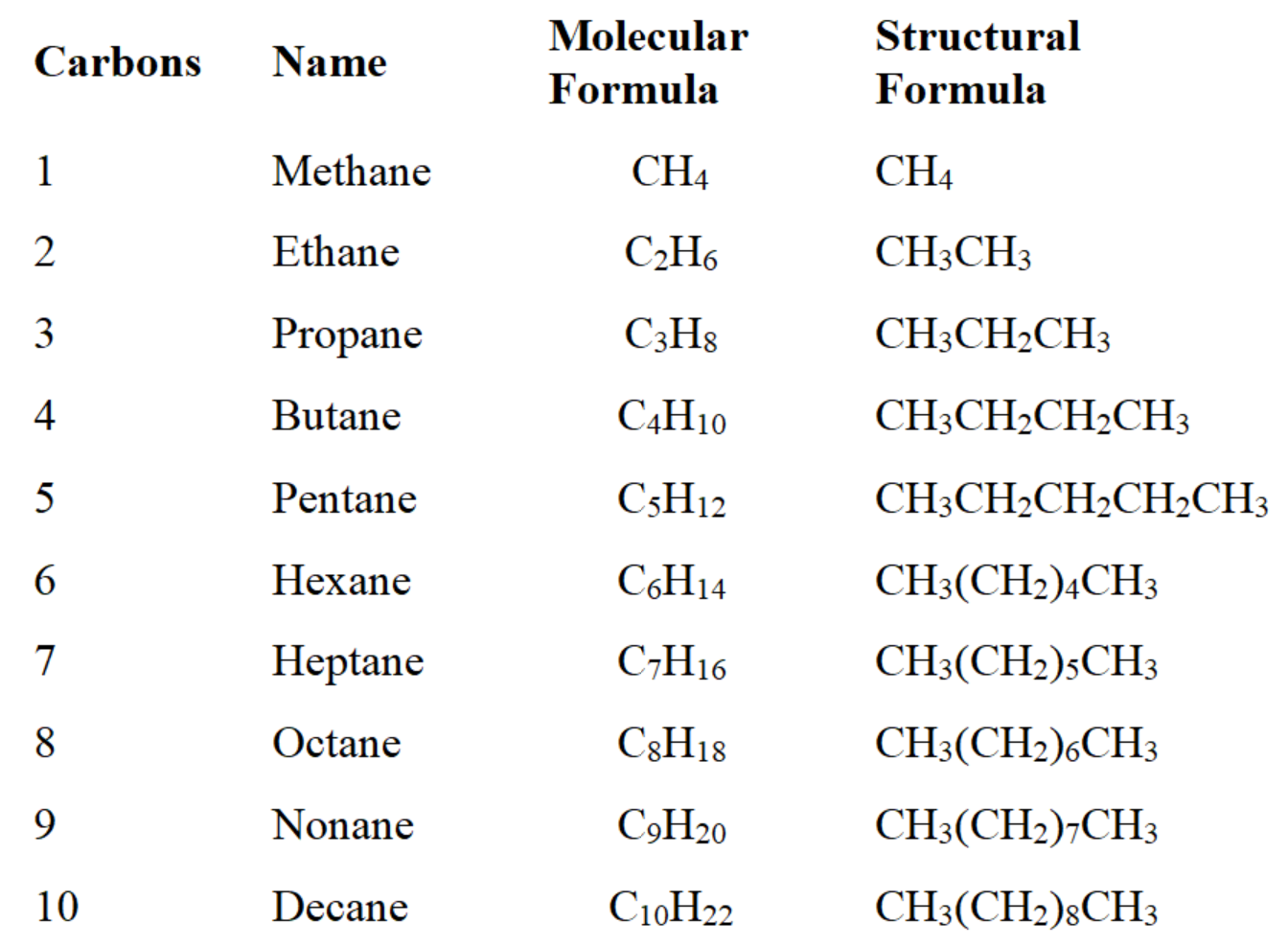
Hydrocarbons
- made of only carbon and hydrogen
- not very functionally useful
- mostly used for energy
- 2 types
- saturated- maximum amount of hydrogens are in the molecule, all carbons have single bonds
- also called alkanes
- unsaturated- some carbons have double or triple bonds
IUPAC Nomenclature
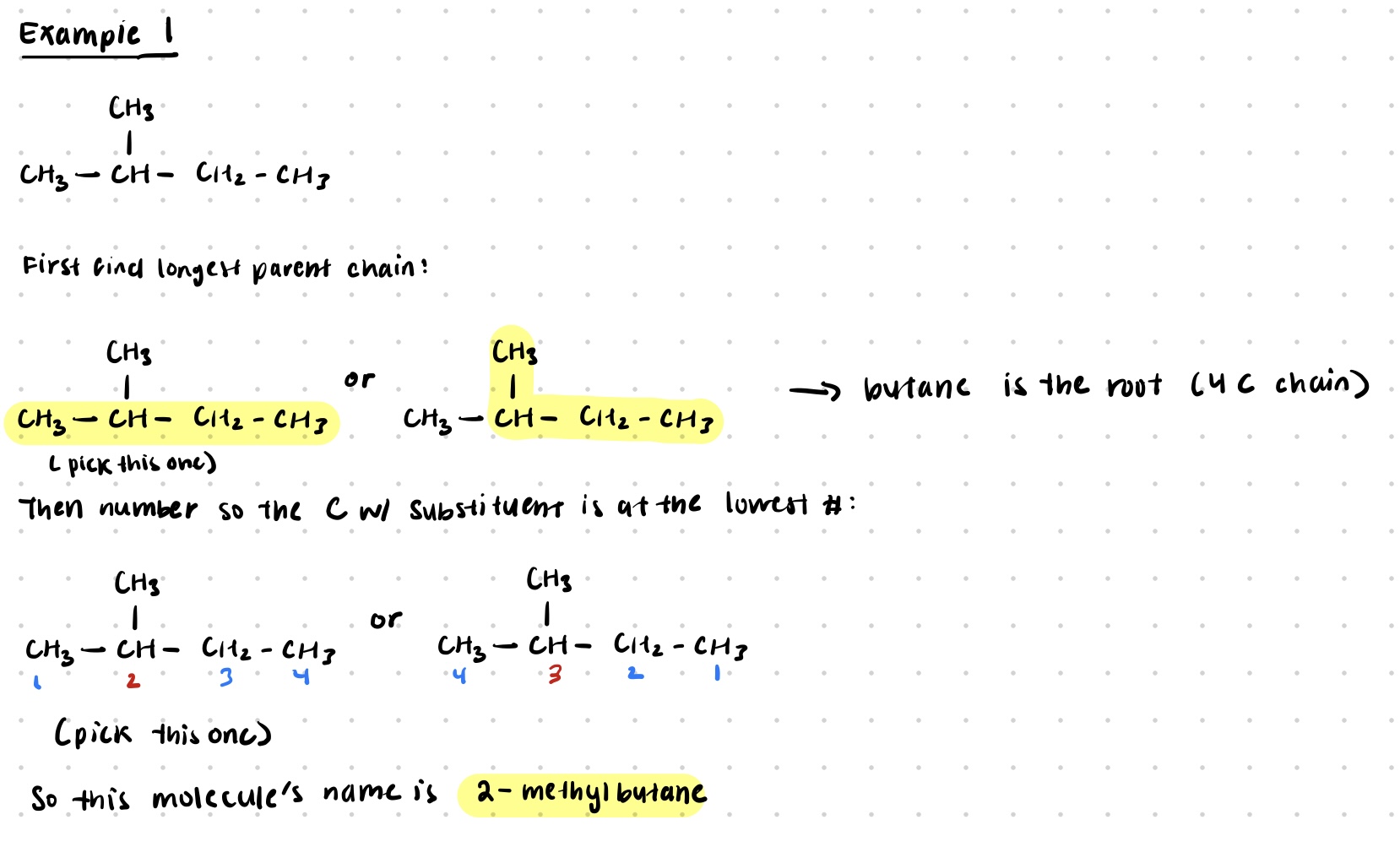
- parent chain is the longest identifiable carbon chain present in the molecule
- if 2 chains have the same length, the parent chain is the one with the most substituents
- carbons in the chain are numbered so the substituents get the lowest number possible
- if some substituents have the same number no matter numbering from left to right, numbering starts from the end where the next substituent has the lowest number
- give the lowest number to the substituent whose letter is first in the alphabet
- if more than one of the same type of substituent is present, use the prefixes di- for 2, tri- for 3, tetra- for 4, etc. to indicate the number
- substituents are listed in alphabetical order
- ignore numerical prefixes in alphabetization (like di-, tri-, tetra-)
- don’t ignore positional prefixes like iso-
- names and numbers are separated by dashes
multiple numbers are separated by commas
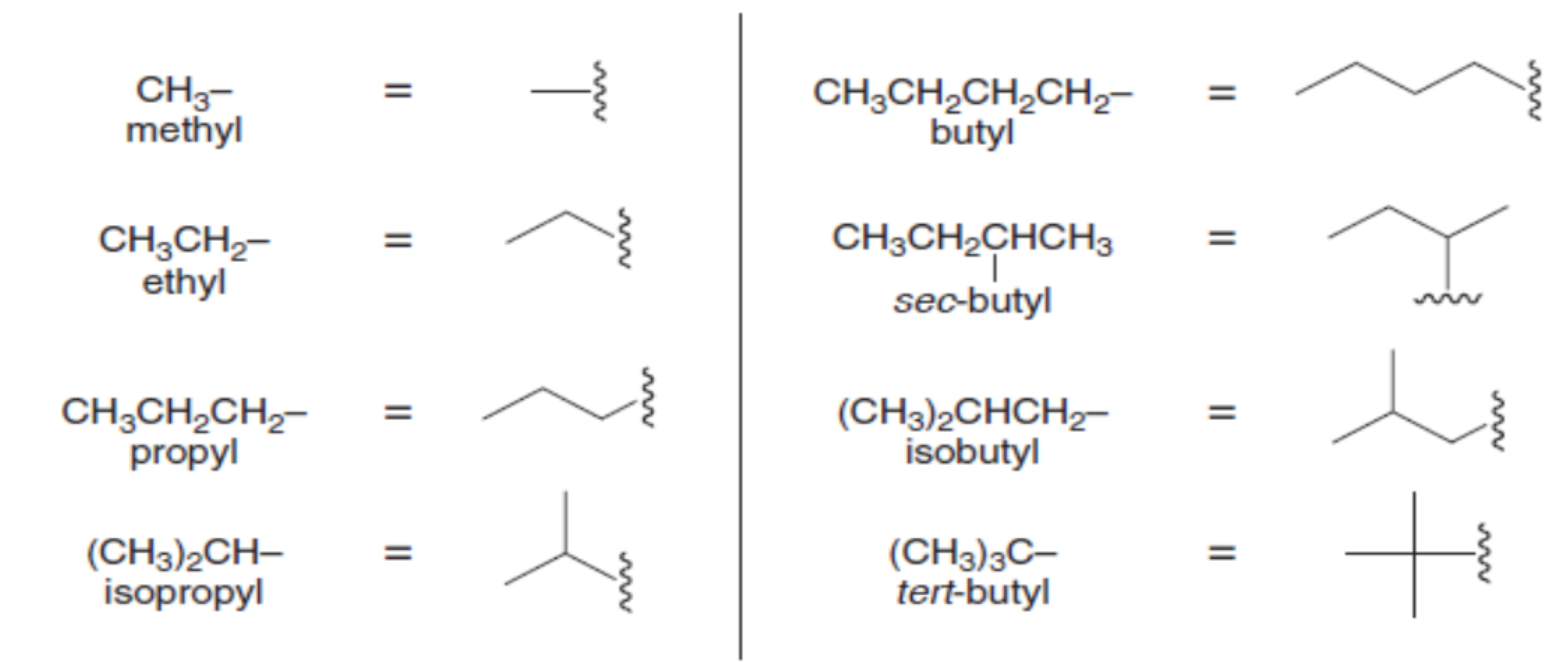
- halogens as substituents
- F: fluoro-
- Cl: chloro-
- Br: bromo-
- I: iodo-
Example