PORG WEEK 13 ALCOHOLS AND PHENOLS
1/71
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
72 Terms
Alcohols
Characterized by one or more hydroxyl groups attached to a carbon atom of a hydrocarbon chain
hydroxyl moiety
Alcohol Alkyl group
–ol
the hydroxyl group is indicated by the ending
saturated, sp3-hybridized carbon atom
In practice, the group name alcohol is restricted to compounds that have their -OH group bonded to a
enols
compounds with their -OH group bonded to a vinylic (-CH=CH2),
sp2-hybridized carbon are called
Carboxylic acid
Aldehyde
Ketone
Alcohol
Amine
Highest to lowest priority
allylic or benzylic
Alcohols are referred to as ______ if the hydroxyl group is bonded to an allylic carbon atom (adjacent to a C=C double bond)
Higher alcohols
those containing 4-10 atoms – are somewhat viscous or oily, they have heavy fruity odors.
solids at room temperature.
Some of the highly branched alcohols containing more than 12 carbon atoms are
higher
The boiling points of alcohols are much _____ than those of alkanes with similar molecular weights
hydrophilic (“water-loving”) group
The hydroxyl group is referred to as a ___________, because it forms hydrogen bonds with water and enhances the solubility of an alcohol in water.
miscible
Methanol, ethanol, n-propyl alcohol, isopropyl alcohol are all _____ with water.
higher
Alcohols with ____ molecular weights tend to be less water-soluble
Methanol
was at one time produced from wood by distillation and is still sometimes called wood alcohol.
Chromium oxide & Zinc oxide
150 standard atmosphere (pressure) at 400°
methanol is manufactured from carbon monoxide and hydrogen using
Methanol
Most of it is used to produce formaldehyde and other chemicals, but some is used as a solvent and as an antifreeze
Methanol
is highly toxic and can cause permanent blindness because when taken internally, it is oxidized to formaldehyde, which binds to opsin, preventing formation of rhodopsin, the light-sensitive pigment needed for vision.
Opsin
universal photoreceptor molecules of all visual systems in the animal kingdom
Rhodopsin
When opsin is damaged, it prevents the formation of ___ which is the light-sensitive pigment needed for vision.
Fomepizole (Antizol ®)
antidote for MeOH/ ethylene glycol poisoning
Formaldehyde
is responsible for carcinogenesis and age-related damage to neurons in the brain
Ethyl alcohol
has been produced mostly through the fermentation of fruit juices. The fermented juice could be stored in a sealed container, and this primitive wine remained safe to drink throughout the winter.
ethanol
is also manufactured by the acid-catalyzed hydration of ethylene.
acid-catalyzed hydration of ethylene
This method, using sulfuric acid or other acid catalysts
Antiseptic
prevent the growth of disease-causing microorganisms.
Nicotinamide adenine dinucleotide
NAD stands for
Disulfiram (Antabuse ®)
used in the treatment of alcohol use disorders by producing unpleasant side effects and sensitivity to alcohol. It is designed as a deterrent to drinking.
80-300mg/dL
Loss of balance and speech, amnesia
300-400mg/dL
N/V, loss of consciousness
400-600mg/dL
Loss of protective reflexes
600mg/dL
Spontaneous respiration, CV problems, death
2-Propanol (isopropyl alcohol)
is manufactured commercially by the acid-catalyzed hydration of propene.
2-Propanol (isopropyl alcohol)
It is the main component of rubbing alcohol and is used in many household and personal care products.
70% isopropyl alcohol
most commonly used disinfectant in pharmaceutical industries. The important thing is that only ________ acts as a disinfectant killing all surface microorganisms. It is used to disinfect hands and equipment surface in pharmaceuticals.
“the glycol made from ethylene.”
The name ethylene glycol refers literally to
ethan-1,2-diol
Ethylene glycol’s systematic name is
Poly(ethylene glycols) (or PEGs)
have been utilized in various forms in pharmaceutical preparations for many years. They are used as additives to creams, as solubilizing agents, and as components of injectable formulations.
Glycerol
is a sweet syrupy substance with three alcohol hydroxyl groups.
Glycerol
was first obtained as a by-product of soap manufacture.
Glycerol
is used in the pharmaceutical industry to prevent the drying of creams and ointments even in cough syrups to help prevent irritation in throats.
Secondary alcohols
are easily oxidized without breaking carbon-carbon bonds only as far as the ketone stage. No further oxidation is seen except under very stringent conditions.
Tertiary alcohols
cannot be oxidized at all without breaking carbon-carbon bonds, whereas primary alcohols can be oxidized to aldehydes or further oxidized to carboxylic acids.
Chromic acid
oxidizes primary alcohols to carboxylic acids, and it oxidizes secondary alcohols to ketones.
Tertiary alcohols
do not react with chromic acid under mild conditions.
Dehydration
are most commonly carried out by warming the alcohol in the presence of a strong dehydrating acid
Esterification of Alcohols
Called as Fischer Esterification
carboxylic esters.
Alcohols can combine with acids to form
Phenols
Any of a family of organic compounds characterized by a hydroxyl group attached to a carbon atom that is a part of an aromatic ring
Phenols
Simplest member, monohydroxybenzene
Phenols
Also known as benzenol or carbolic acid
Monohydroxy compounds
are only slightly soluble in water but are miscible with organic solvents.
Joseph Lister
In 1865 the British surgeon _____ used phenol as an antiseptic to sterilize his operating field.
Phenol
is quite toxic, however, and concentrated solutions cause severe but painless burns of the skin and mucous membranes.
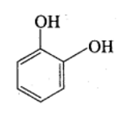
Catechol
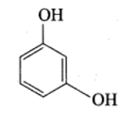
Resorcinol
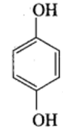
Quinol
Pyrogallol
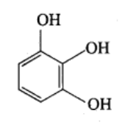
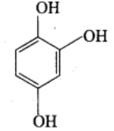
Hydroxyquinol
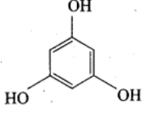
Phloroglucinol
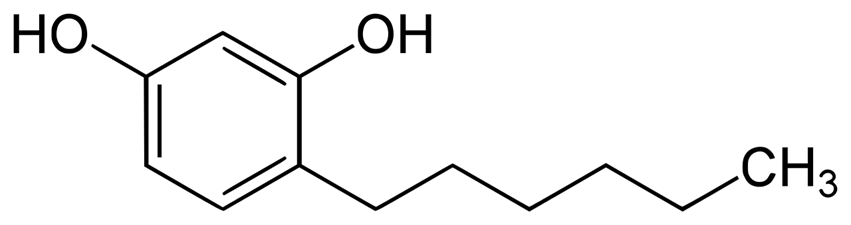
n-hexylresorcinol
Less-toxic phenols, such as ____-, have supplanted phenol itself in cough drops and other antiseptic applications
Butylated hydroxytoluene (BHT)
has a much lower toxicity and is a common antioxidant in foods.
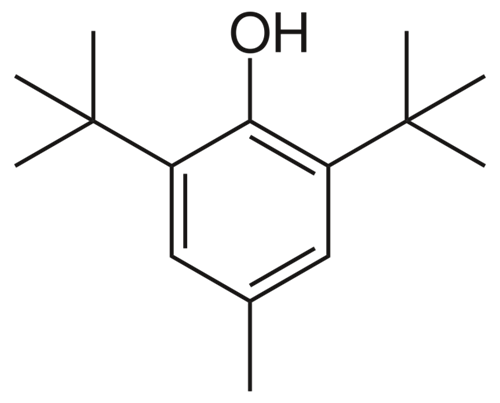
Tyrosine
One of the standard amino acids found in most proteins
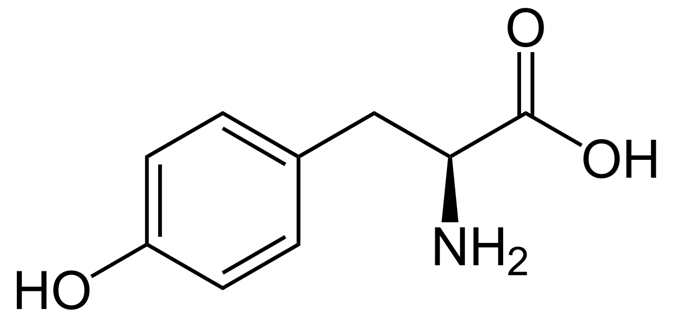
Epinephrine
AKA adrenaline
Stimulant hormone produced by the adrenal medulla
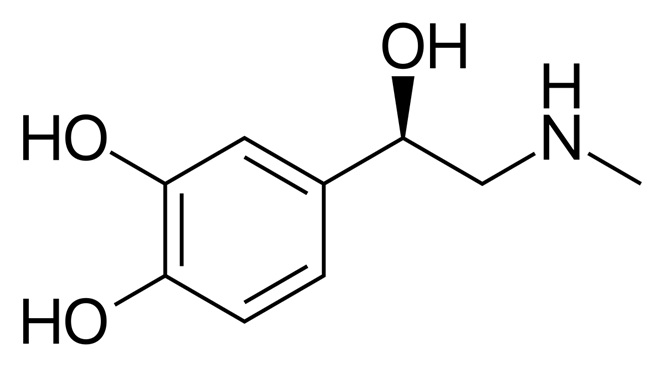
Serotonin
A neurotransmitter in the brain
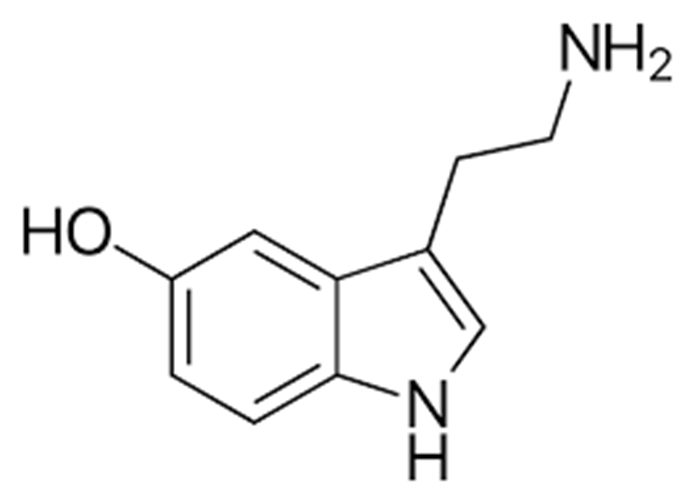
Urushiol
An irritant secreted by poison ivy to prevent animal from eating its leaves
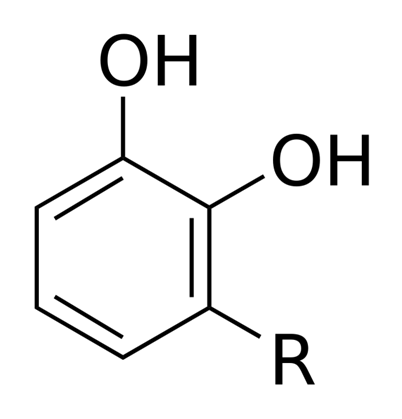
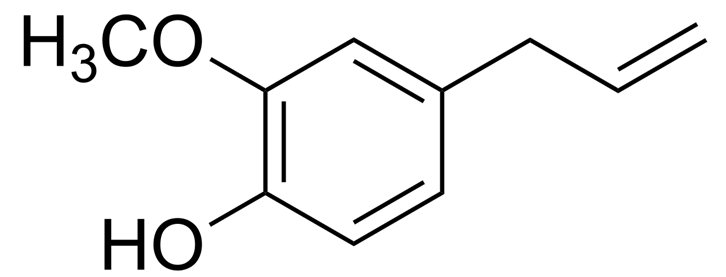
Vanillin
the principal flavouring in vanilla, is isolated from vanilla beans
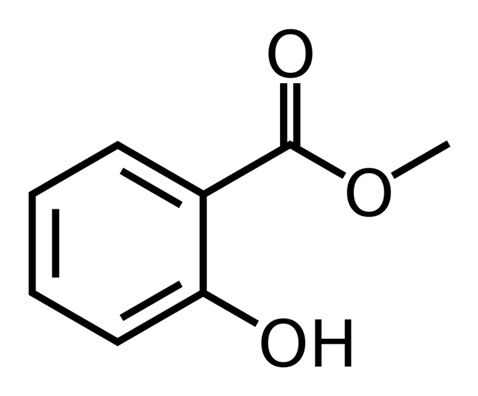
Methyl Salicylate
has a characteristic minty taste and odour, is isolated from wintergreen
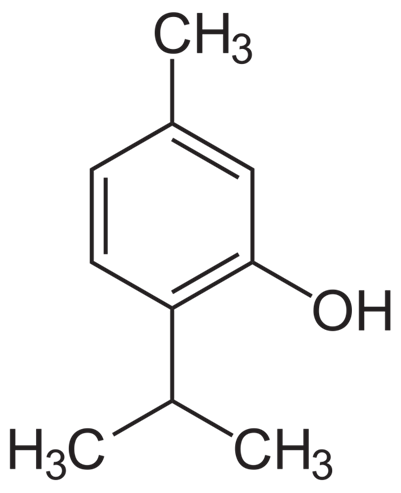
Thymol
Extracted from Thyme
fungicidal
Eugenol
Extracted from clove oil
Antiseptic in mouthwashes
Dental analgesic