chemical substances formulas and names
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
Chemical formula
a notation that uses atomic symbols with numerical subscripts to convey the relative proportions of atoms of the different elements in the substance
Molecule
A molecule is a definite group of atoms that are chemically bonded together that is tightly connected by attractive forces
Molecular substance
A molecular substance is a substance that is composed of molecules, all of which are alike
Polymers
are very large molecules that are made up of a number of smaller molecules repeatedly linked together
Monomers
are the small molecules that are linked together to form the polymer
Structural formula
A structural formula is a chemical formula that shows how the atoms are bonded to one another in a molecule
Polyatomic ion
A polyatomic ion is an ion consisting of two or more atoms chemically bonded together and carrying a net electric charge
Ion
An ion is an electrically charged particle obtained from an atom or chemically bonded group of atoms by adding or removing electrons .N
Naming of ionic compounds
name an ionic compound by giving the name of the cation followed by the name of the anion
Rules for naming monatomic ions
Monatomi cations are named after the element if there is only one such ion. Example Al3+ is called aluminium ion.
If there is more than one monatomic cation of an element , rule 1 is not sufficient , the stock system of nomenclature names the cations after the element as in rule 1 but follows this by a roman numeral in parentheses denoting the charge on the ion. Example Fe2+ is called iron (ll) ion and Fe3+ is called iron (lll) ion. In an older system of nomenclature, ions are named by addng the suffixes -ous and -ic to a stem name of the element to indicate the ions and higher charge ,respectively. Example : Fe2+ is calle ferrous ion , Fe3+ ferric ion
Naming of binary compounds (Ionic compounds)
name of cation first then anion
metal atom retains the name of the element , when the element can form cations with different charges , the charge is written as a Roman numeral in brackets
name of non-metals is changed to end in -ide
EXAMPLE: CaBr2 is calcium bromide not calcium (ll) bromide or calcium dibromide because calcium can only form the Ca2+ cation an bromine only the Br anion Cr2O3 is chromium (lll) oxide
Molecular (covalent) compounds
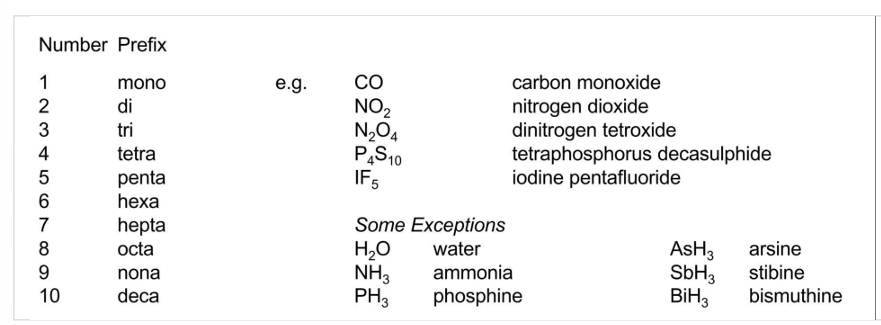
Equation
symbolic representation of a chemical reaction in terms of chemical formulas
CN-
cyanide
OH-
hydroxide
C2H3O2-
acetate
CIO4-
perchlorate
CIO3-
chlorate
CIO2-
Chlorite
CIO-
Hypochlorite
NO3-
nitrate
NO2-
nitrite
CO3 minus 2 charge
carbonate
CrO4 -2 charge
chromate
SO4 2- charge
sulfate
SO3 2- charge
sulfite
PO4 -3 charge
phosphate
PO3 -3 charge
phosphite