4. Ligand-Gated Ion Channels
1/48
Earn XP
Description and Tags
complete
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
What are the essential functions of ion channels (3)?
Transport ions across membrane e.g. secretion/absorption of fluids.
Regulate membrane potentials e.g. Nerve and muscle cells for high-speed communication.
Ca2+ influx into the cytoplasm e.g. secretion and muscle contraction.
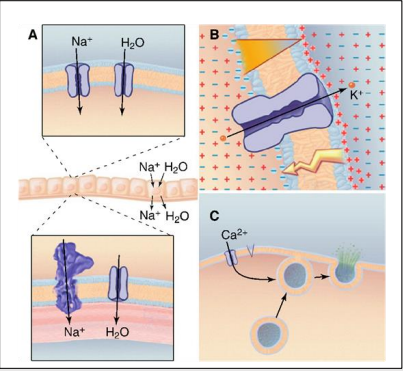
What is a transmembrane domain?
Protein that spans the width of the membrane from the extracellular to intracellular sides usually a helical shape.
What is a P-loop or pore?
Pocket where ion will bind.
What are the main structural features of ion channels?
Transmembrane proteins made of 2 or more α-helices crossing the lipid bilayer.
Composed of 2-6 subunits surrounding the ion-conducting pore.
Classified by:
Gating mechanism
Ion selectivity, based on the physical size of the pore and amino acids lining it.
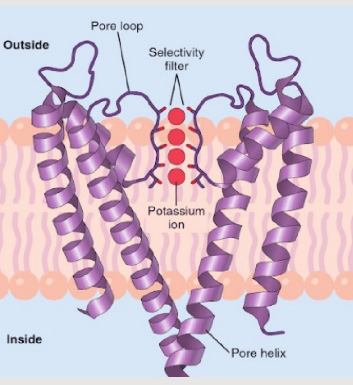
Which ion channel serves as a model for all ion channels?
The pH-regulated K+ channel (KcsA) from Streptomyces lividans serves as a model for all ion channels.
What is the structure of a simple K+ ion channel?
Where are TMs more tightly packed?
What controls the gate?
Transmembrane helicases (TM) form a p-loop (pore) which is highly selective based on size & charge.
On the cytoplasmic side, TMs are tightly packed, creating a gate.
Factors controlling the gate:
Membrane potential
Mechanical stress
Ligands
What are the main functions of voltage-gated ion channels?
Na+ and K+ create action potentials in excitable cells.
Ca2+ enters the cytoplasm, acting as a second messenger to elicit cellular responses.
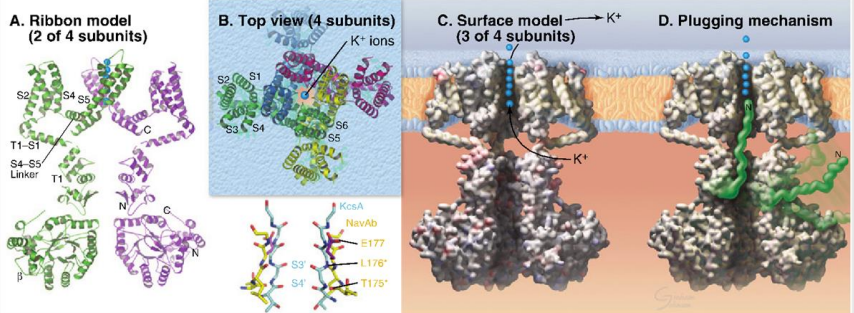
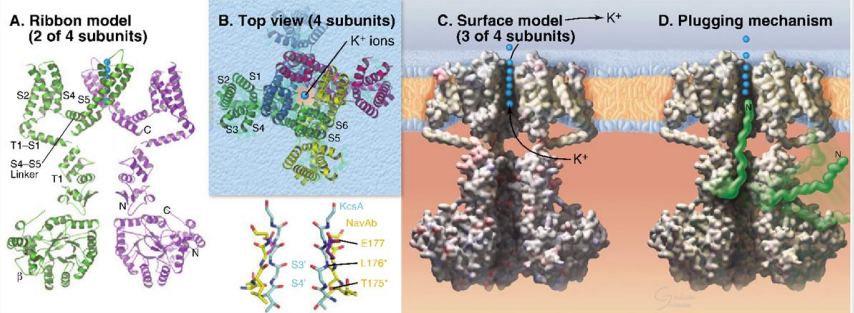
What are the main structural features of voltage-gated ion channels?
Similar to simple ion channels but with additional helices S1 to S4.
S1 and S4 form a voltage-sensing domain.
Large polypeptides extend into the cytoplasm, with a plugging mechanism to control ion flow.
Transient Receptor Potential (TRP) Channels
What are they structurally similar to?
Where are they found?
What are they involved in (2)?
Share common structural features with voltage-gated channels BUT evolved to sense chemicals and physical stimuli.
Found in sensory neurons.
Involved in thermosensing and nociception (pain sensing).
What controls ligand-gated ion channels?
Ligand-gated ion channels are controlled by the binding of a ligand (instead of voltage changes).
Intracellularly (inside the cell).
Extracellularly (outside the cell).
What are 2 examples of intracellular ligand-gated ion channels?
Cyclic nucleotide-binding domain.
Calmodulin bound to the N-terminal.
What are ionotropic receptors?
Ionotropic receptors are families of channels gated by extracellular ligands, which control ion flow across the membrane.
What do Na+/K+ selective channels control?
Na+/K+ selective channels control membrane excitability and help to depolarize cells.
How do calcium-permeable channels affect cellular activity?
Channels with added permeability to calcium can directly regulate the activity of calcium-sensitive proteins.
What is the role of Cl- selective channels in cellular activity?
Cl- selective channels control membrane excitability by reducing resistance, causing hyperpolarization, and reducing action potential firing.
What type of assembly do Cys-loop type receptors have?
Cys-loop type receptors have a pentameric assembly, meaning they are made up of 5 subunits.
Give an example of a Cys-loop type receptor.
The nicotinic acetylcholine receptor (nAChR) is an example of a Cys-loop type receptor.
How many subunits does the nicotinic acetylcholine receptor (nAChR) in muscle have, and what are they?
The nAChR in muscle is composed of 5 subunits: ⍺, β, 𝛾, and ε.
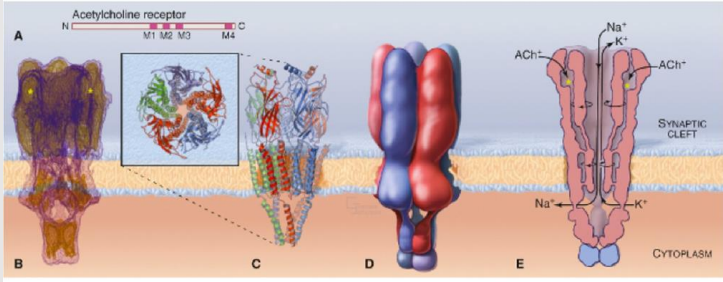
What are the structural features of each subunit of the nAChR?
Each subunit of nAChR has 4 transmembrane segments (M1, M2, M3, M4), a large external-facing N domain, and an intracellular loop between M3 and M4.
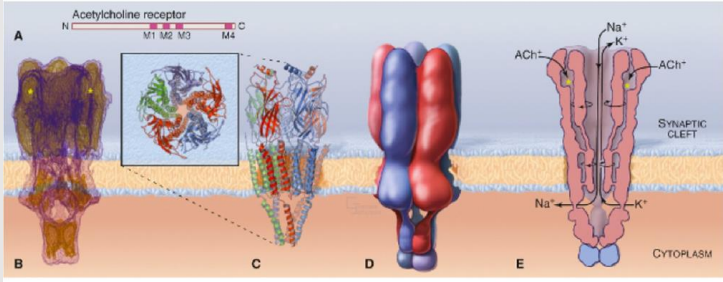
Describe the subunits within ligand-gated ion channel families.
Multiple subunits exist within any one ligand-gated ion channel family.
How do different subunit combinations affect ligand-gated ion channels and what does this provide?
Different subunit combinations make up receptors in different parts of the brain, providing diversity in function. This complexity causing diversity provides opportunity for drug targeting.
What role does the nACh⍺4 subunit play in the brain?
The nACh⍺4 subunit is involved in reward pathways and nicotine addiction.
What types of neuronal nAChRs exist?
Neuronal nAChRs exist as α2 - 10 and β2 - 4, each having different affinities depending on their composition and location.
Where are α4β2 receptors primarily expressed, and what is their significance?
α4β2 receptors are abundantly expressed in the cortex and hippocampus, and they have a high affinity for agonists such as nicotine and varenicline.
What happens to nicotinic receptors with chronic exposure to nicotine?
Chronic exposure leads to receptor upregulation.
What genetic studies have revealed about nicotine dependence?
Genetic studies have shown specific polymorphisms in subunit genes CHRNA4 (α4) and CHRNA6 (α6) that are linked to tobacco dependence.
What have studies found about rare variants in nicotinic receptor genes?
Rare variants have been shown to be protective against nicotine dependence.
What condition is caused by mutations in the nAChR?
Autosomal Dominant Nocturnal Frontal Lobe Epilepsy (ADNFLE).
Which specific region of the human α4 neuronal nicotinic subunit is associated with Autosomal Dominant Nocturnal Frontal Lobe Epilepsy (ADNFLE)?
Mutations in the M2 region of the human α4 neuronal nicotinic subunit cause ADNFLE.
How many mutations have been identified in relation to ADNFLE?
Nine mutations have been identified to date.
What effect do mutations in nAChR have on receptor function?
Mutations cause use-dependent potentiation and a delay in the rising phase due to slow unblocking of closed receptors, leading to enhanced receptor function.
How does enhanced nAChR function contribute to ADNFLE seizures?
Enhanced receptor function leads to increased nicotinic-mediated transmitter release, which is associated with ADNFLE seizures.
What is the main neurotransmitter in the brain?
Glutamate
What is the structural organization of glutamate receptors?
Glutamate receptors are tetrameric, forming as a dimer of dimers.
How is the pore structure of glutamate receptors different from other channels like KcsA?
The pore of glutamate receptors is inverted compared to the KcsA structure.
Where is the ligand binding site located in glutamate receptors, and what happens when it is occupied?
The ligand binding site is in a cleft that closes when occupied.
Why are glutamate receptors important in the brain?
Glutamate receptors are vital to every aspect of brain function and dysfunction, contributing to various human diseases.
What mechanisms contribute to the diversity of glutamate receptors?
Multiple genes, alternative splicing, and RNA editing contribute to the diversity in pharmacology, permeability, and function of glutamate receptors.
What is the primary function of AMPA receptors?
AMPA receptors mediate fast excitatory synaptic transmission in the central nervous system.
What is the role of NMDA receptors, and how do they compare to other glutamate receptor isoforms?
NMDA receptors are involved in learning and memory and have slower synaptic transmission than other isoforms.
What are Kainate receptors, and what conditions are they linked to?
Kainate receptors function similarly to AMPA but play a lesser role at synapses. They are linked to schizophrenia, depression, and Huntington’s disease.
What are the two RNA splicing isoforms of each AMPA receptor subunit, and how do they differ?
The two RNA splicing isoforms are flip and flop.
Flop has a faster desensitization rate and reduced current responses to glutamate compared to flip.
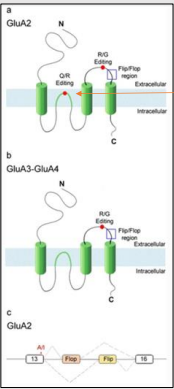
What is the significance of RNA splicing in AMPA receptors?
Alternative splicing of two exons creates two protein isoforms (flip & flop) with different extracellular domains, resulting in different kinetic properties of the receptor.
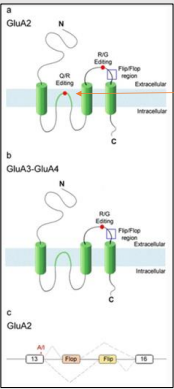
What is RNA editing at the GluA2 Q/R site, and where is it located?
The GluA2 Q/R site is in the M2 region of the subunit, inside the channel pore. RNA editing changes a CAG (glutamine) codon to a CGG (arginine) codon.
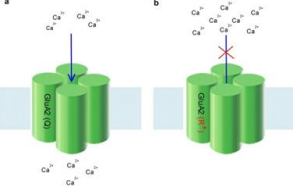
What is the effect of RNA editing at the GluA2 Q/R site on AMPA receptor Ca2+ permeability?
RNA editing at the GluA2 Q/R site reduces Ca2+ permeability of the channel. Unedited receptors have increased Ca2+ permeability.
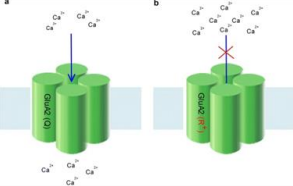
What are the effects of lacking the enzyme responsible for RNA editing at the GluA2 Q/R site in mice?
Mutant mice lacking the RNA editing enzyme are prone to seizures and experience early death.
NMDA receptor
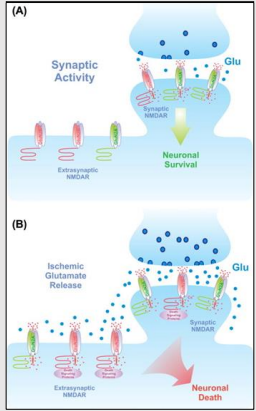
What is the primary function of the NMDA receptor in the brain?
What happens when the NMDA receptor is excessively stimulated, such as in a stroke?
The NMDA receptor is crucial for controlling synaptic plasticity and mediating learning and memory functions.
Excessive stimulation of the NMDA receptor during a stroke can lead to neuron death.
Downregulation of GluA2 Q/R editing
What is the consequence of downregulation of GluA2 Q/R editing in ALS patients' motor neurons?
What causes the downregulation of GluA2 Q/R editing in ALS patients?
How is decreased ADAR2 activity in glioblastoma correlated with malignancy?
How can GluA2 Q/R editing impact glioblastoma treatment?
It leads to an increase in Ca2+ permeable AMPA receptors, causing damage due to glutamate excitotoxicity.
It is caused by the downregulation of the editing enzyme ADAR2, not a mutation in the editing site.
Decreased ADAR2 activity correlates with increased malignancy by raising Ca2+ levels, which activates the Akt pathway, promoting proliferation and tumorigenesis.
When GluA2 Q/R editing was restored, the increased Ca2+ levels and Akt pathway activation were reversed, suggesting potential therapeutic applications.
P2X receptors
What ligand gates P2X receptors?
What is the structural composition of P2X receptors?
How many ATP molecules are required to open the P2X receptor channel?
What are some P2X receptor associated diseases/physiological conditions?
Adenosine triphosphate (ATP) gates P2X receptors.
Composed of 3 subunits with 2 transmembrane helices and a large extracellular domain. Have 7 subtypes. P2X 1 - 7.
Three ATP molecules are needed to open the channel.
Hearing loss, pain, inflammation, neurodegenerative disease.