Dalton's Atomic Theory and 3 Laws
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
Postulate 1
All matter consists of atoms. Atoms are tiny indivisible particles of an element that cannot be created or destroyed.
Postulate 2
Atoms of one element cannot be converted into atoms of another element.
Postulate 3
All atoms of a given element have the same mass and other properties that distinguish them from other atoms.
OR YOU CAN DESCRIBE IT LIKE THIS:
Atoms of an element are identical in mass and other properties and are different from the atoms of any other element.
Postulate 4
Compounds result from the chemical combination of a specific ratio of atoms of different elements.
ORR YOU CAN DEFINE IT THIS WAY:
Atoms of different elements combine to form compounds in simple, whole-number ratios
Law of Mass
The total mass of substance does not change during a chemical reaction.
Law of Definite (or Constant) Composition
States that no matter the source, a particular compound always contains the same elements in the exact same proportions by mass (fractions)
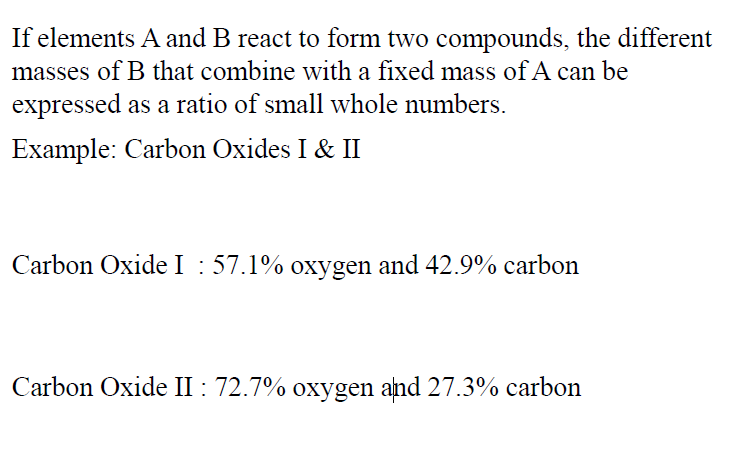
Law of Multiple Proportions
When different numbers of atoms of elements combine, they
must do so in ratios of small, whole numbers. (this is referring to the same element that can combine")
[The law of multiple proportions states that whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers.]
TEXTBOOK DEFINITION: PICTURE
![<p>When different numbers of atoms of elements combine, they</p><p>must do so in ratios of small, whole numbers. (this is referring to the same element that can combine")</p><p>[The law of multiple proportions states that whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers.]</p><p></p><p>TEXTBOOK DEFINITION: PICTURE</p>](https://knowt-user-attachments.s3.amazonaws.com/8769a98f-7b84-4dd7-a7c0-072d64cace32.png)