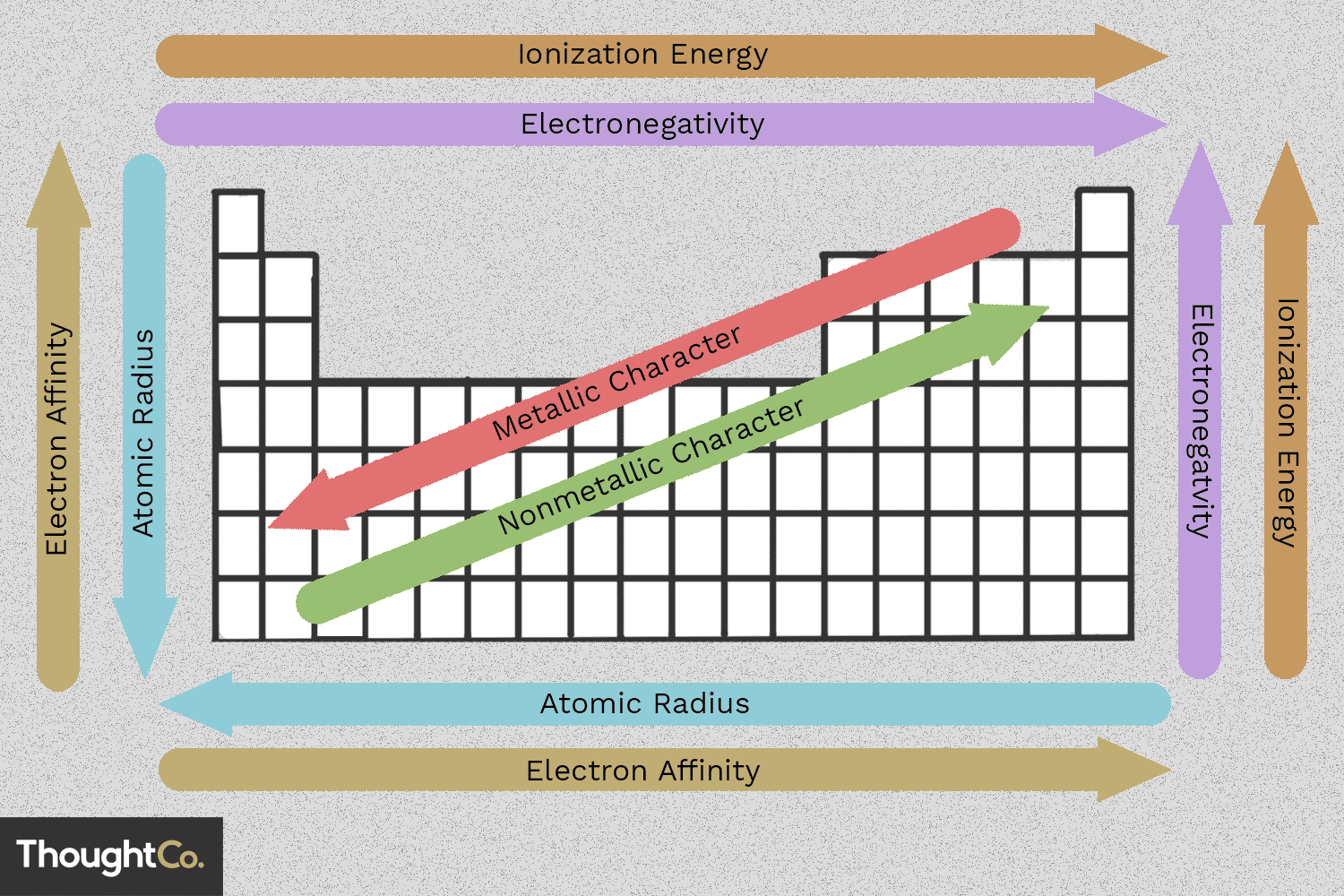
3.6 - Periodic Trends
Terms
Atomic Radius: distance between the nucleus and the valence level of electrons
Electronegativity: measure of the ability of an atom to attract additional electrons to itself
Ionization Energy: energy required to remove electrons from an atom
Electron Affinity: energy given when an atom gains an electron
↓ Group Trends ↓
INCREASING energy levels
INCREASING shielding effect
- so therefore -
INCREASING atomic radius
→Period Trends→
INCREASING # protons
INCREASING nuclear charge
SAME energy level
SAME shielding effect [constant]
- so therefore -
DECREASING atomic radius
ELECTRONEGATIVITY AND IONIZATION ENERGY VARIES