BIO 311C - SATA - EXAM 1
1/116
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
117 Terms
Why study cellular and molecular biology?
The connecting basis of all life is at the cellular and molecular level. DNA, RNA, proteins and the cellular mechanisms behind each of these form the fundamental basis of life. Many biological phenomena are better understood at the biochemical and molecular level.
What are the 8 emergent properties of life?
Reproduction
Growth and Development
Order and Structure
Metabolism
Respiration
Response to environmental stimuli
Adaptation and evolution
Autonomous movement
What is a model system?
Because there are millions of living organisms, and we cannot study each one individually, so we use these selected organisms to study the mechanisms of life. It is a representative organism or cell type used for experiments. They are easy to grow, manipulate, and study.
What are some common examples of model systems among prokaryotes?
- E. coli
- Salmonella
What are some common examples of model systems among eukaryotes - plants?
• Arabidopsis
• Corn
• Rice
What are some common examples of model systems among eukaryotes - fungi?
• Yeast
What are some common examples of model systems among eukaryotes - animals?
• Fruit fly
• Nematode
• Mouse
• Zebra fish
• HeLa cells (human cell lines)
In vivo
Experiments used to study physiology, ecology of organisms under living conditions.
Ex: rats, rabbits, plant tissue culture, etc.
Can be holistic or reductionist
In vitro
Experiments performed under non-living AKA abiotic condition
Ex: in a test tube with known quantities of chemical, enzymes added, particular temperature, pH, etc.
Strictly reductionist
What is the biological hierarchy?
Atoms →Molecules→Macromolecules→Parts of cells→Cells→Tissues→Organs→Organ systems→Organisms→Population→Community→Ecosystem→Biome→Biosphere
Name the 3 domains and 4 kingdoms.
1) Bacteria
2) Archaea
3 Eukarya
- Protista
- Fungi
- Plantae
- Animalia
mass number
# protons + # neutrons
molecular weight
sum of the weight of all atoms in a molecule
expressed in Daltons (Da)
valence
Refers to an atom's bonding capacity and is determined by how many unpaired valence electrons there are out of 8.
Covalent bonds
Occur when two atoms share a pair of electrons, stronger IMF
Polar Covalent bonds
Unequal sharing of electrons
Ex: H2O, NH3
Non-polar Covalent bonds
Equal sharing of electrons
Ionic bonds
Occurs when the electronegativity of one atom is greater than that of the adjoining atom, the one with higher electronegativity pulls electrons to its valence shell from its neighboring atom, thereby becoming an anion. The atom it took the electron from becomes a cation. The ionic bonding occurs because of the attraction between the positive and negative charges.
H - bonds
Hydrogen covalently attached to one electronegative atom is attracted to another electronegative atom.
Van der Waals interactions
Takes place when molecules interact; there are pockets of constantly changing positive and negative charges. This takes place due to the changing distribution of electron clouds and results in weak forces of attraction.
Hydrophilic interactions
Polar, charged molecules
Soluble
Hydrophobic interactions
Non-polar
lipid- soluble
Excluded from aqueous solutions bc of hydrogen bonding among polar molecules
Biological example of Covalent bonds
Carbohydrates - glycosidic bonds
Lipids - ester bonds
Proteins - peptide bonds
DNA/RNA - phosphodiester bonds
Biological example of Ionic bonds
NaCl
Biological example of H-bonds
H2O, NH3
DNA
RNA
Proteins
Biological example of Van der Waals interactions
Lipids in biological membranes
Cellulose in plant walls
Biological example of Hydrophilic/Hydrophobic interactions
Phospholipids in biological membranes have a hydrophilic region outside the membrane bilayer, hydrophobic region within
Amino acids with hydrophobic side chains are in the interior of the protein while those with hydrophilic side chains can be in the interior or the exterior
What is the relation between the properties of water and H-bonding? How do they help support life on earth?
Water molecules have two partial positive and two partial negative charges, allowing for 4 counts of hydrogen bonding, and thus water molecules can bond with up to four other water molecules. The H-bonding is strong in water and is the main reason for its unique properties.
Properties of Water
cohesiveness
adhesiveness
high specific heat
high heat of vaporization
freezing and expansion
versatile solvency
medium for biochemical reactions
ingredient of many biochemical reactions
cohesiveness
due to the constant forming and breaking of H-bonds in liquid water, H2O molecules stick together
- important for water uptake ( transport from roots ) and imbibition ( water absorption by seeds )
adhesiveness
water can adhere to surfaces
- important for the attachment of water ( adsorption ), which leads to absorption
high specific heat
1 cal/g/C°
Water can absorb and release heat to stabilize the temperature in the surrounding area, making it habitable.
high heat of vaporization
it takes lots of energy to evaporate water, which is important in evaporative cooling in animals and plants in hot weather.
freezing and expansion
as water freezes, it expands, becoming less dense; the resulting ice floats on the top of the water, keeping water below warmer, and thus providing a favorable environment for aquatic organisms during freezing cold conditions
versatile solvent
water is an excellent solvent for all polar and charged molecules because of its polarity
minerals and nutrients dissolved in water are available for easy uptake and transport by plants and animals.
medium for biochemical reactions
living cells contain up to 95% water so many biochemical reactions in a cell occur under aqueous conditions
ingredient of many biochemical reactions
many biochemical reactions need water as a reactant
Ex: photosynthesis, hydrolysis
How to prepare solutions of different molar concentrations when given a molecular weight, percent weight, volume solution, etc.
MW (g/mol) X M (mol/L) x L = g
From stock solution to a diluted solution
C1V1 = C2V2
C1 - concentration of stock solution
V1 - volume of stock solution
pH
pH > 7 base
pH = 7 neutral
pH < 7 acid
Kw
water constant = 10^-14
As pH increases
[H+] decreases
[OH-] increases
becomes more basic
A solution with a pH of 4 has ____ x more [H+] that one with a pH of 6
100
pK
when acid and base have a ratio of 1:1
mid point of buffering range
buffer
weak acid/base/both substance that minimizes pHchange by donating or accepting protons
example of acid
stomach acid
example of weak base
blood
example of strong base
bleach
Organic molecules
carbon
hydrogen
nitrogen
oxygen
phosphorus
sulfur
structural isomer
same molecular formula but different structures
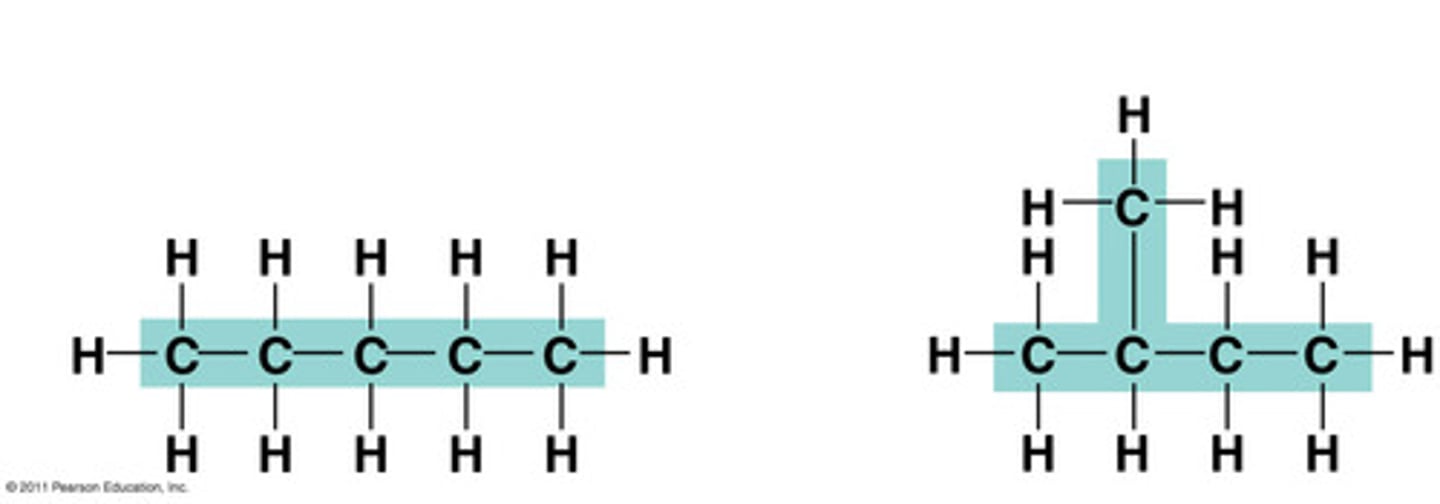
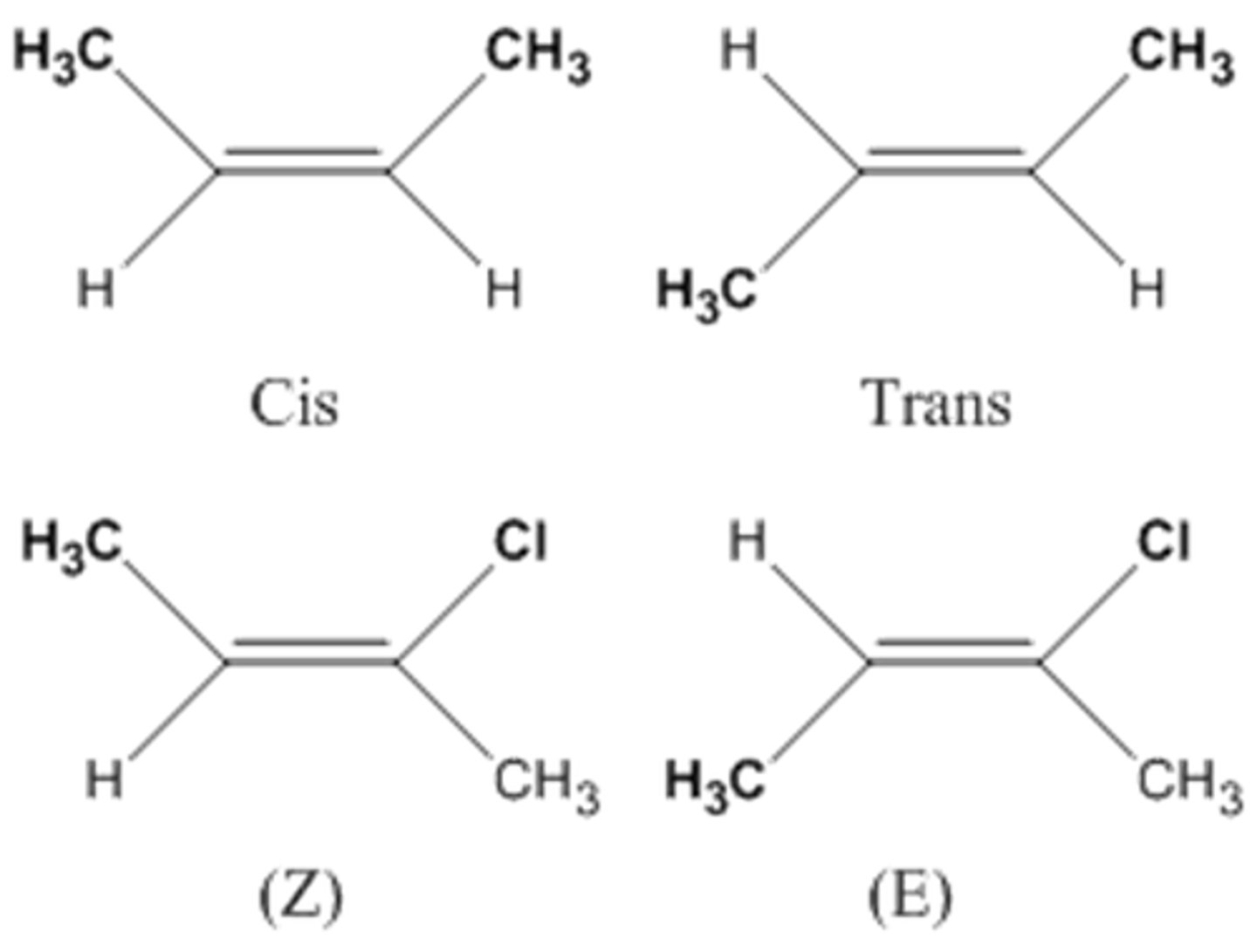
geometric isomer
form due to inflexibility of double bonds between carbons
cis - same types of functional groups on the same side
trans - same types of functional groups away from each otehr
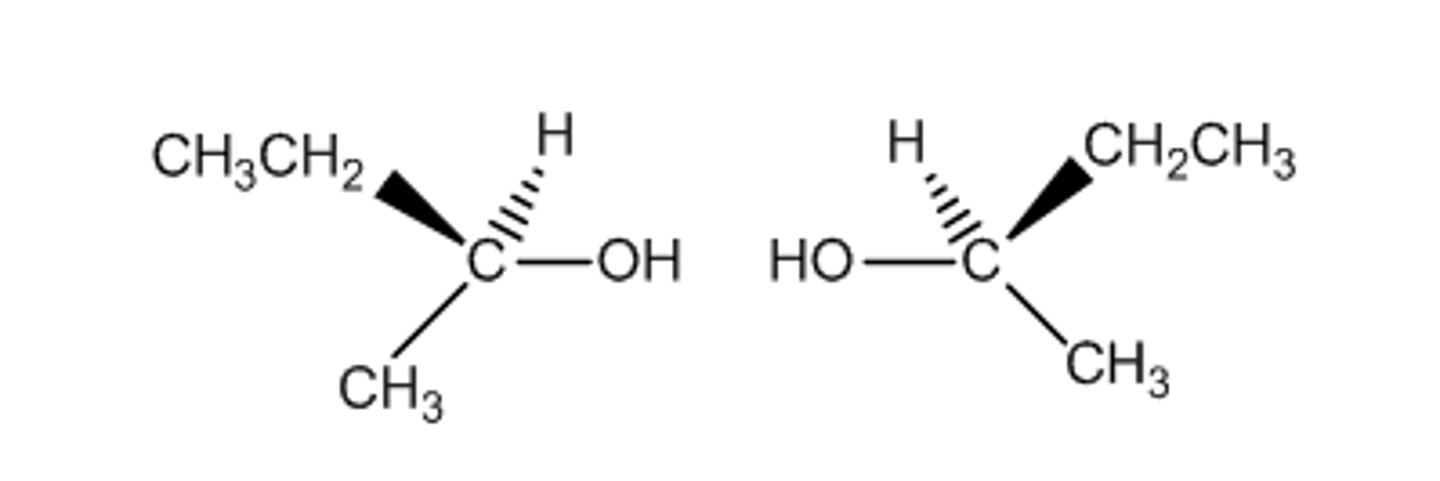
optical isomer
also called enantiomers
mirror images
Functional groups
hydroxyl
hydroxyl
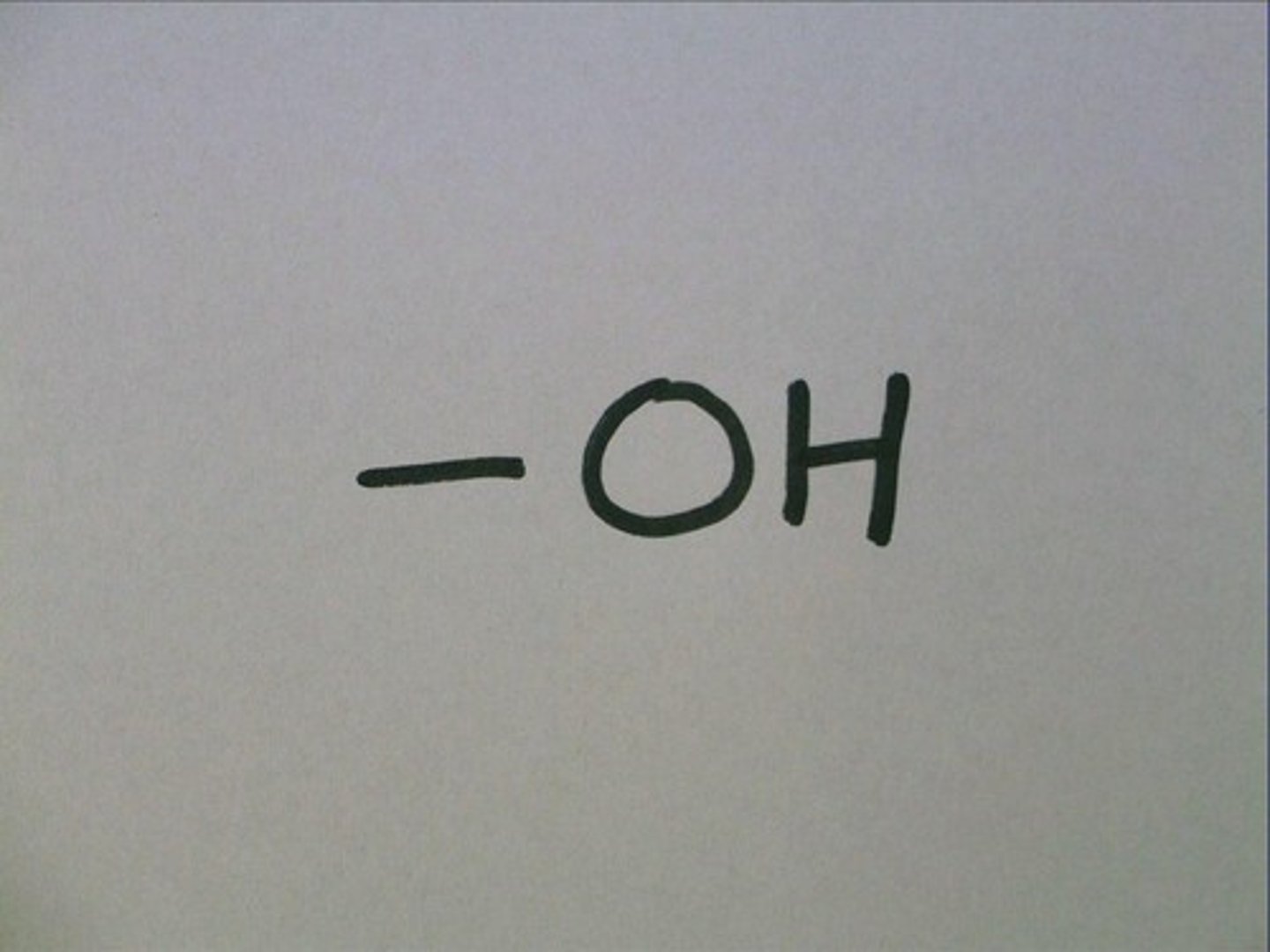
hydroxyl
confers polarity to a molecule, making it more soluble in water
hydroxyl
almost all carbs, proteins, nucleic acids have these
hydroxyl
molecules with these groups are called alcohols
carbonyl
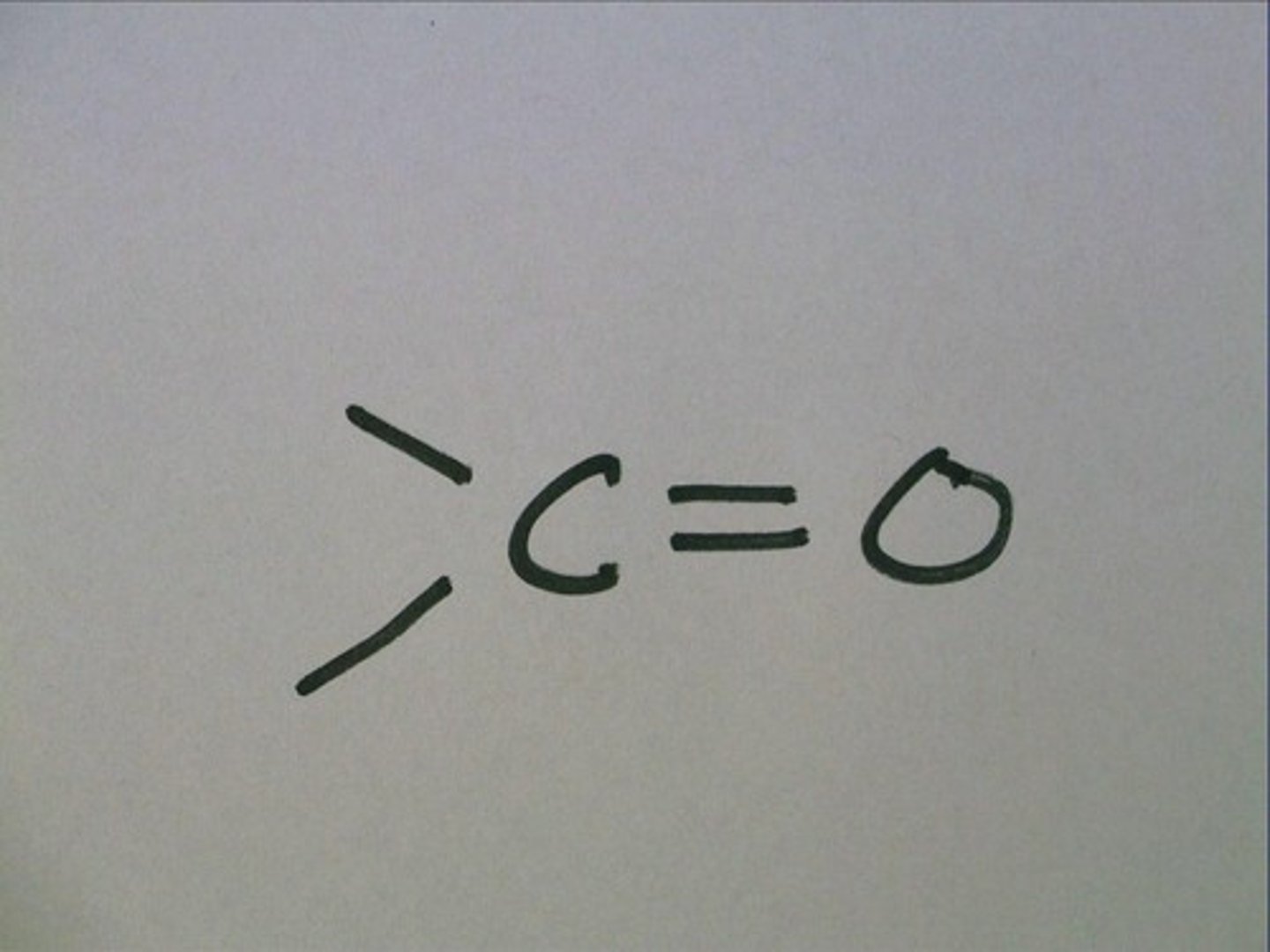
if the carbonyl is at the end of an organic molecule it is
an aldehyde (glucose)
if the carbonyl is in the middle of an organic molecule it is
a ketone (fructose)
carbonyl
slightly polar
carbonyl
present in simple sugars, some proteins and nucleotides
carboxyl
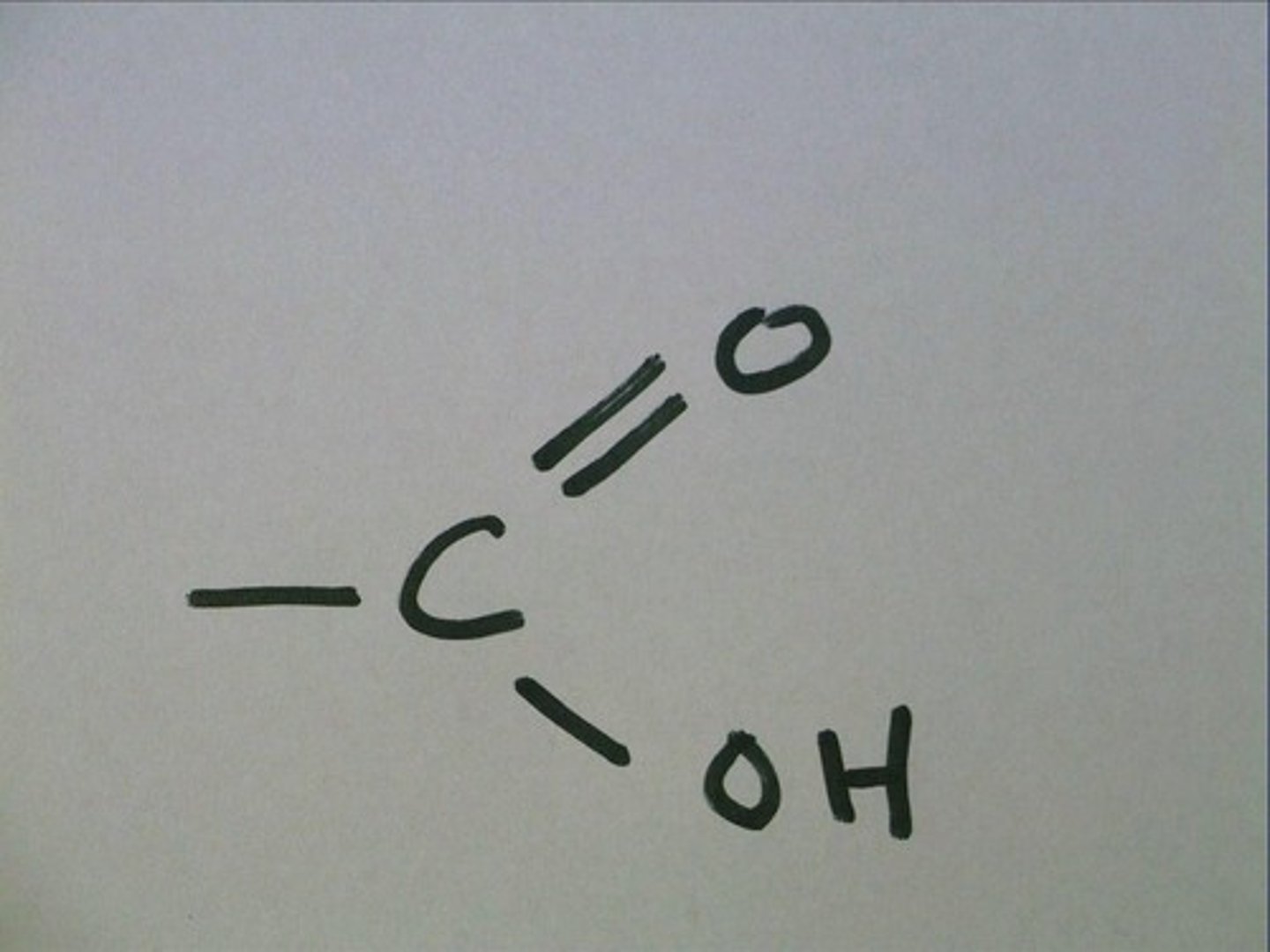
carboxyl
an acidic group that ionizes to form -COO- and H+ to increase [H+] of a solution
carboxyl
acetic acid, formic acid, citric acid, malic acid
ionized by this group to become
acetate, formate, citrate, malate
carboxyl
present in all amino acids and proteins
amino group
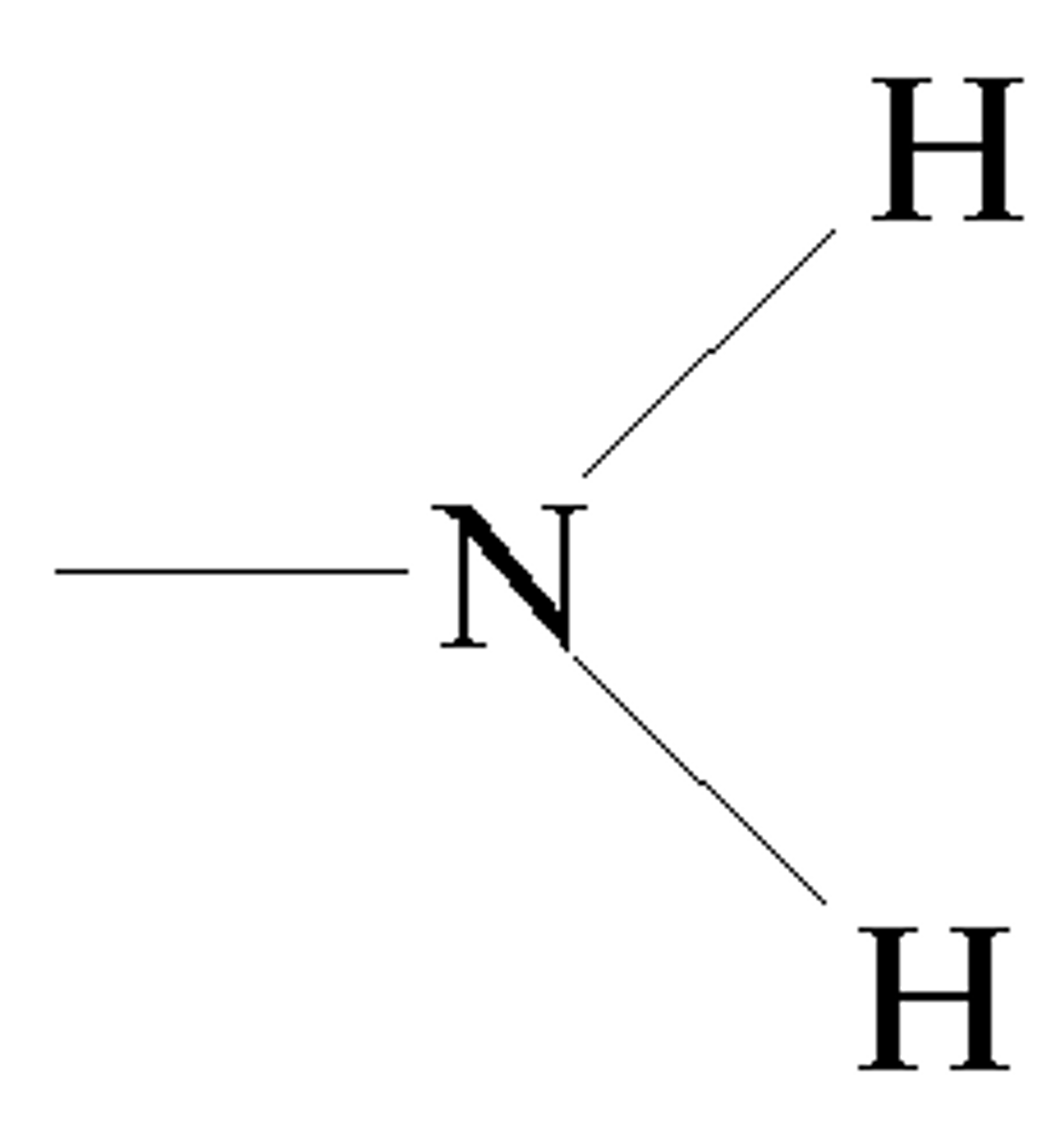
amino group
can act as a base by accepting protons
(-NH2 + H+ = -NH3+)
amino group
found in all amino acids, proteins, and some specific nucleotides
sulfhydryl
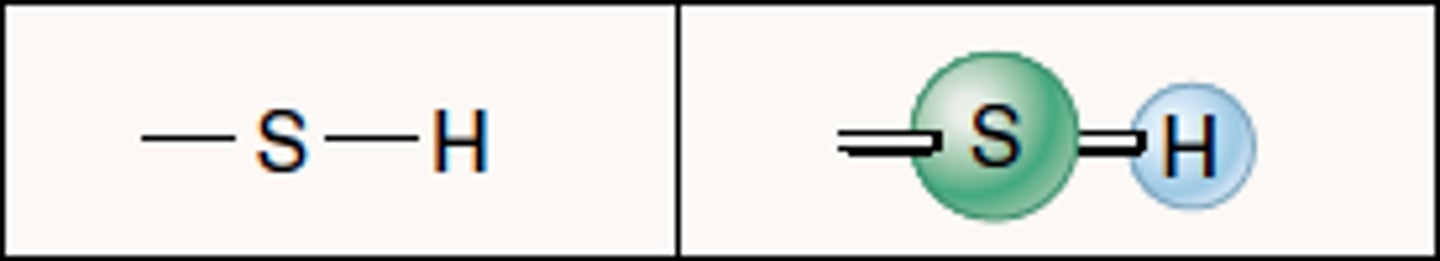
sulfhydryl
two of these groups within a protein can combine to make a disulfide bridge to stabilize a protein structure
sulfhydryl
found in active sites involved in the catalysis of enzymes
phosphate
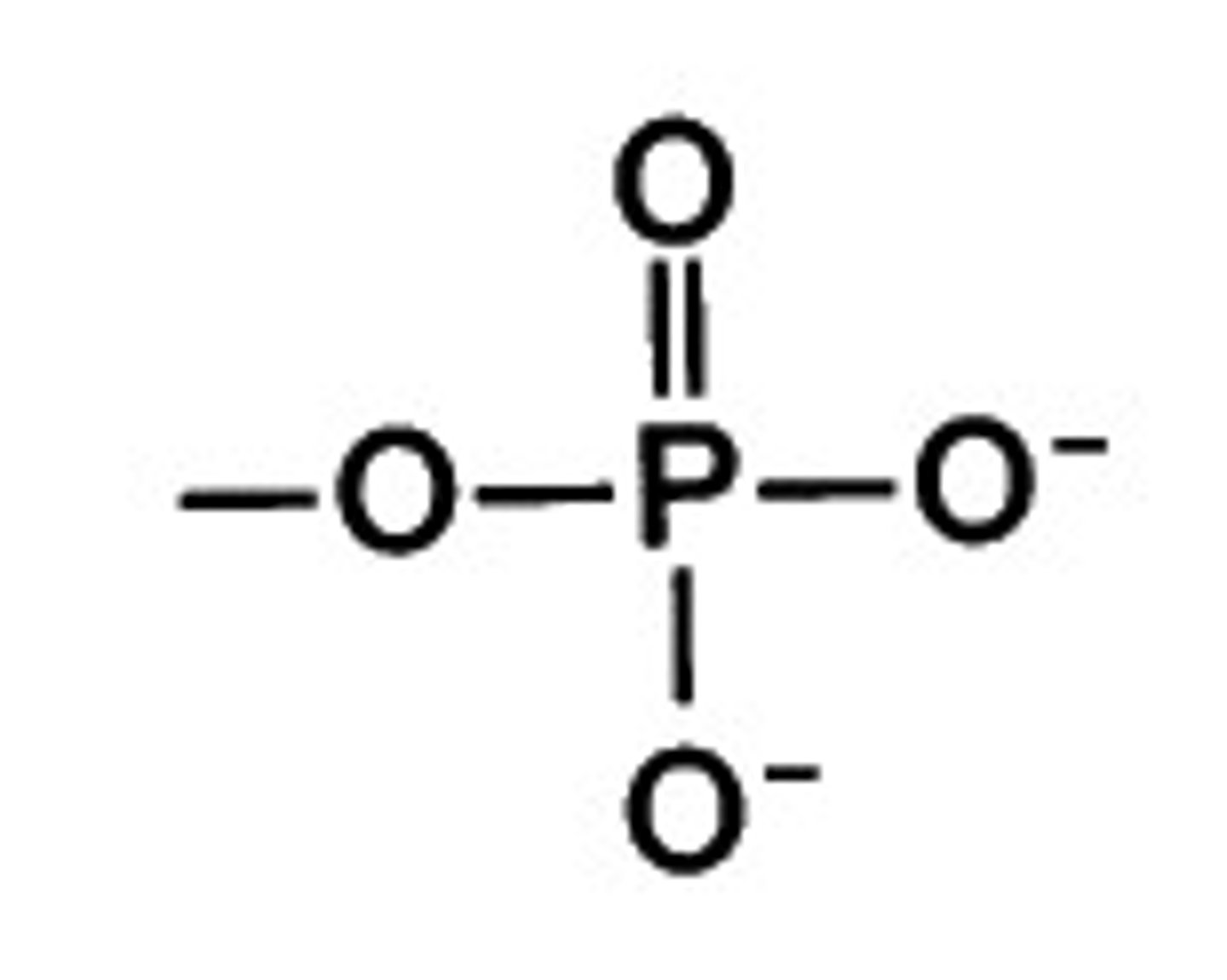
phosphate
highly polar
phosphate
important in energy compounds such as ATP
phosphate
present in DNA, RNA, and all nucleotides
phosphate
found in phospholipids
phosphate
acidic and reactive group
methyl
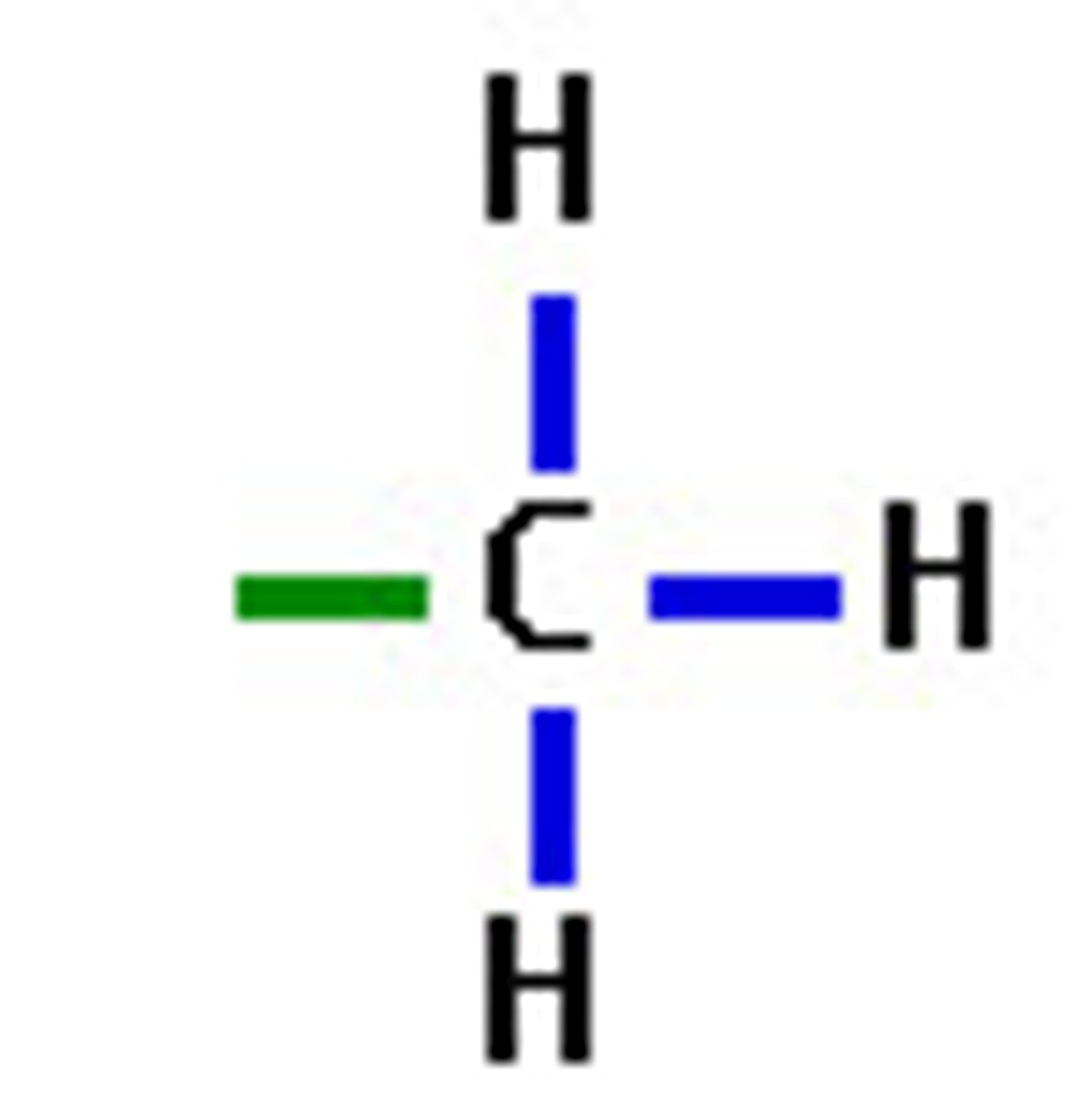
methyl
non-polar functional group
methyl
affect the solubility of a compound in aqueous or organic solutions
methyl
strong influence on the bioactivity of the molecule involved
methylation
adding a methyl group to a DNA molecule can make it non-functional
methylation
this makes somedrugs and pesticides more permeable through cell membranes
methyl
found in alcohols, fatty acids, some amino acids, and nucleotides
Four major groups of biological molecules
carbohydrates
lipids
proteins
nucleic acids
condensation synthesis
combines two monomers and releases a water molecule by removing an -H and -OH group
synthesizes polymers
hydrolysis
breaks down polymers into monomers by adding an -H and -OH group by splitting a water molecule
hydrolysis
important for catabolic processes
Carbohydrate monomers
monosaccharides
Simple sugars are made up of
C, H, and O
In carbs, C:H:O
1:2:1
Carbon skeleton size variation
3 to 7 carbons
Within a carbon skeleton
an -OH group is attached to each C, except for one which is double-bonded to an O (carbonyl-CO)
In aqueous solutions, sugars with _ to _ carbons form a ring structure
5, 7
Monosaccharide (sugar) function
provides a major source of energy for cells
energy stored in sugars is harvested by cells through respiration
carbon skeletons are used for making other molecules
Disaccharide
formed by enzymes which combine two monosaccharides through glycosidic linkages
maltose
glucose + glucose
lactose
glucose + galactose
sucrose
glucose + fructose